An Alternative Approach to Detecting Cancer Cells by Multi-Directional Fluorescence Detection System Using Cost-Effective LED and Photodiode
- PMID: 31109061
- PMCID: PMC6566952
- DOI: 10.3390/s19102301
An Alternative Approach to Detecting Cancer Cells by Multi-Directional Fluorescence Detection System Using Cost-Effective LED and Photodiode
Abstract
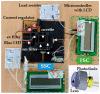
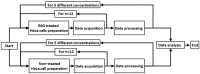
The enumeration of cellular proliferation by covering from hemocytometer to flow cytometer is an important procedure in the study of cancer development. For example, hemocytometer has been popularly employed to perform manual cell counting. It is easily achieved at a low-cost, however, manual cell counting is labor-intensive and prone to error for a large number of cells. On the other hand, flow cytometer is a highly sophisticated instrument in biomedical and clinical research fields. It provides detailed physical parameters of fluorescently labeled single cells or micro-sized particles depending on the fluorescence characteristics of the target sample. Generally, optical setup to detect fluorescence uses a laser, dichroic filter, and photomultiplier tube as a light source, optical filter, and photodetector, respectively. These components are assembled to set up an instrument to measure the amount of scattering light from the target particle; however, these components are costly, bulky, and have limitations in selecting diverse fluorescence dyes. Moreover, they require multiple refined and expensive modules such as cooling or pumping systems. Thus, alternative cost-effective components have been intensively developed. In this study, a low-cost and miniaturized fluorescence detection system is proposed, i.e., costing less than 100 US dollars, which is customizable by a 3D printer and light source/filter/sensor operating at a specific wavelength using a light-emitting diode with a photodiode, which can be freely replaceable. The fluorescence detection system can quantify multi-directional scattering lights simultaneously from the fluorescently labeled cervical cancer cells. Linear regression was applied to the acquired fluorescence intensities, and excellent linear correlations (R2 > 0.9) were observed. In addition, the enumeration of the cells using hemocytometer to determine its performance accuracy was analyzed by Student's t-test, and no statistically significant difference was found. Therefore, different cell concentrations are reversely calculated, and the system can provide a rapid and cost-effective alternative to commercial hemocytometer for live cell or microparticle counting.
Keywords: cancer cells; fluorescence detection system; light-emitting diode; photodiode.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
A low-cost light-emitting diode induced fluorescence detector for capillary electrophoresis based on an orthogonal optical arrangement.Talanta. 2009 May 15;78(3):1155-8. doi: 10.1016/j.talanta.2009.01.033. Epub 2009 Jan 24. Talanta. 2009. PMID: 19269486
-
Fluorescence detector for capillary separations fabricated by 3D printing.Anal Chem. 2014 Dec 16;86(24):11951-6. doi: 10.1021/ac503678n. Epub 2014 Nov 26. Anal Chem. 2014. PMID: 25427247
-
Flexible planar microfluidic chip employing a light emitting diode and a PIN-photodiode for portable flow cytometers.Lab Chip. 2012 Jan 7;12(1):197-203. doi: 10.1039/c1lc20672a. Epub 2011 Nov 15. Lab Chip. 2012. PMID: 22086498
-
Fluorescent Biosensors Based on Single-Molecule Counting.Acc Chem Res. 2016 Sep 20;49(9):1722-30. doi: 10.1021/acs.accounts.6b00237. Epub 2016 Sep 1. Acc Chem Res. 2016. PMID: 27583695 Review.
-
Flow cytometry: basic principles and applications.Crit Rev Biotechnol. 2017 Mar;37(2):163-176. doi: 10.3109/07388551.2015.1128876. Epub 2016 Jan 14. Crit Rev Biotechnol. 2017. PMID: 26767547 Review.
Cited by
-
Automatic Cancer Cell Taxonomy Using an Ensemble of Deep Neural Networks.Cancers (Basel). 2022 Apr 29;14(9):2224. doi: 10.3390/cancers14092224. Cancers (Basel). 2022. PMID: 35565352 Free PMC article.
-
A Novel Multistage Transfer Learning for Ultrasound Breast Cancer Image Classification.Diagnostics (Basel). 2022 Jan 6;12(1):135. doi: 10.3390/diagnostics12010135. Diagnostics (Basel). 2022. PMID: 35054303 Free PMC article.
References
-
- Absher M. Tissue Culture. Elsevier; Amsterdam, The Netherlands: 1973. Hemocytometer counting; pp. 395–397.
-
- Kaushik S. Magnetic Flow Cytometry for Point-of-Care Applications. [(accessed on 18 May 2019)]; Available online: https://cloudfront.escholarship.org/dist/prd/content/qt45b287bv/qt45b287....
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

