Neuroprotective Effects of Diets Containing Olive Oil and DHA/EPA in a Mouse Model of Cerebral Ischemia
- PMID: 31109078
- PMCID: PMC6566717
- DOI: 10.3390/nu11051109
Neuroprotective Effects of Diets Containing Olive Oil and DHA/EPA in a Mouse Model of Cerebral Ischemia
Abstract
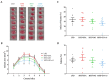
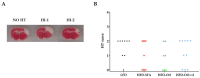
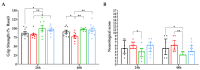
Stroke is one of the leading causes of death worldwide and while there is increasing evidence that a Mediterranean diet might decrease the risk of a stroke, the effects of dietary fat composition on stroke outcomes have not been fully explored. We hypothesize that the brain damage provoked by a stroke would be different depending on the source of dietary fat. To test this, male C57BL/6J mice were fed for 4 weeks with a standard low-fat diet (LFD), a high-fat diet (HFD) rich in saturated fatty acids (HFD-SFA), an HFD containing monounsaturated fatty acids (MUFAs) from olive oil (HFD-OO), or an HFD containing MUFAs from olive oil plus polyunsaturated fatty acids (PUFAs) docosahexaenoic acid/eicosapentaenoic acid (DHA/EPA) (HFD-OO-ω3). These mice were then subjected to transient middle cerebral artery occlusion (tMCAo). Behavioural tests and histological analyses were performed 24 and/or 48 h after tMCAo in order to elucidate the impact of these diets with different fatty acid profiles on the ischemic lesion and on neurological functions. Mice fed with HFD-OO-ω3 displayed better histological outcomes after cerebral ischemia than mice that received an HFD-SFA or LFD. Furthermore, PUFA- and MUFA-enriched diets improved the motor function and neurological performance of ischemic mice relative to those fed with an LFD or HFD-SFA. These findings support the use of DHA/EPA-omega-3-fatty acid supplementation and olive oil as dietary source of MUFAs in order to reduce the damage and protect the brain when a stroke occurs.
Keywords: DHA; EPA; MUFA; PUFA; SFA; behaviour; cerebral ischemia; middle cerebral artery occlusion (MCAo); neurological function; neuroprotection; olive oil; omega-3 fatty acids; stroke.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures



Similar articles
-
Docosahexaenoic acid reduces resting blood pressure but increases muscle sympathetic outflow compared with eicosapentaenoic acid in healthy men and women.Am J Physiol Heart Circ Physiol. 2019 Apr 1;316(4):H873-H881. doi: 10.1152/ajpheart.00677.2018. Epub 2019 Feb 8. Am J Physiol Heart Circ Physiol. 2019. PMID: 30735073 Clinical Trial.
-
Dietary DHA/EPA Ratio Changes Fatty Acid Composition and Attenuates Diet-Induced Accumulation of Lipid in the Liver of ApoE-/- Mice.Oxid Med Cell Longev. 2018 Nov 14;2018:6256802. doi: 10.1155/2018/6256802. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30538803 Free PMC article.
-
The Omega-3 Fatty Acids EPA and DHA, as a Part of a Murine High-Fat Diet, Reduced Lipid Accumulation in Brown and White Adipose Tissues.Int J Mol Sci. 2019 Nov 24;20(23):5895. doi: 10.3390/ijms20235895. Int J Mol Sci. 2019. PMID: 31771283 Free PMC article.
-
Impact of the Co-Administration of N-3 Fatty Acids and Olive Oil Components in Preclinical Nonalcoholic Fatty Liver Disease Models: A Mechanistic View.Nutrients. 2020 Feb 15;12(2):499. doi: 10.3390/nu12020499. Nutrients. 2020. PMID: 32075238 Free PMC article. Review.
-
It Is Time for an Oil Change: Polyunsaturated Fatty Acids and Human Health.Mo Med. 2021 Sep-Oct;118(5):426-430. Mo Med. 2021. PMID: 34658434 Free PMC article. Review.
Cited by
-
Integrating multi-omics with neuroimaging and behavior: A preliminary model of dysfunction in football athletes.Neuroimage Rep. 2021 Jul 15;1(3):100032. doi: 10.1016/j.ynirp.2021.100032. eCollection 2021 Sep. Neuroimage Rep. 2021. PMID: 40567291 Free PMC article.
-
Short Term Usage of Omega-3 Polyunsaturated Fatty Acids Ameliorate Lipopolysaccharide-Induced Inflammatory Response and Oxidative Stress in the Neonatal Rat Hippocampal Tissue.Front Nutr. 2020 Nov 17;7:572363. doi: 10.3389/fnut.2020.572363. eCollection 2020. Front Nutr. 2020. PMID: 33282898 Free PMC article.
-
The Polyunsaturated Fatty Acid EPA, but Not DHA, Enhances Neurotrophic Factor Expression through Epigenetic Mechanisms and Protects against Parkinsonian Neuronal Cell Death.Int J Mol Sci. 2022 Dec 19;23(24):16176. doi: 10.3390/ijms232416176. Int J Mol Sci. 2022. PMID: 36555817 Free PMC article.
-
Pien-Tze-Huang attenuates neuroinflammation in cerebral ischaemia-reperfusion injury in rats through the TLR4/NF-κB/MAPK pathway.Pharm Biol. 2021 Dec;59(1):828-839. doi: 10.1080/13880209.2021.1942926. Pharm Biol. 2021. PMID: 34196587 Free PMC article.
-
Stearic acid methyl ester affords neuroprotection and improves functional outcomes after cardiac arrest.Prostaglandins Leukot Essent Fatty Acids. 2020 Aug;159:102138. doi: 10.1016/j.plefa.2020.102138. Epub 2020 May 23. Prostaglandins Leukot Essent Fatty Acids. 2020. PMID: 32663656 Free PMC article.
References
-
- Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67. doi: 10.1161/CIR.0000000000000558. - DOI - PubMed
-
- James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
-
- World Health Organization. [(accessed on 25 February 2019)]; Available online: https://www.who.int/topics/cerebrovascular_accident/en/
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

