Reconsidering the Role of Cyclooxygenase Inhibition in the Chemotherapeutic Value of NO-Releasing Aspirins for Lung Cancer
- PMID: 31109107
- PMCID: PMC6572483
- DOI: 10.3390/molecules24101924
Reconsidering the Role of Cyclooxygenase Inhibition in the Chemotherapeutic Value of NO-Releasing Aspirins for Lung Cancer
Abstract
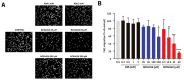
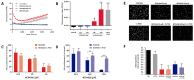
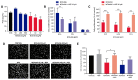
Nitric oxide-releasing aspirins (NO-aspirins) are aspirin derivatives that are safer than the parent drug in the gastrointestinal context and have shown superior cytotoxic effects in several cancer models. Despite the rationale for their design, the influence of nitric oxide (NO•) on the effects of NO-aspirins has been queried. Moreover, different isomers exhibit varying antitumor activity, apparently related to their ability to release NO•. Here, we investigated the effects and mode of action of NO-aspirins in non-small-cell lung cancer (NSCLC) cells, comparing two isomers, NCX4016 and NCX4040 (-meta and -para isomers, respectively). NCX4040 was more potent in decreasing NSCLC cell viability and migration and exhibited significant synergistic effects in combination with erlotinib (an epidermal growth factor receptor inhibitor) in erlotinib-resistant cells. We also studied the relationship among the effects of NO-aspirins, NO• release, and PGE2 levels. NCX4040 released more NO• and significantly decreased PGE2 synthesis relative to NCX4016; however, NO• scavenger treatment reversed the antiproliferative effects of NCX4016, but not those of NCX4040. By contrast, misoprostol (a PGE2 receptor agonist) significantly reversed the antiproliferative effect of NCX4040, but not those of NCX4016. Furthermore, misoprostol reversed the antimigratory effects of NCX4040. Overall, these results indicate that PGE2 inhibition is important in the mode of action of NO-aspirins.
Keywords: NCX4016; NCX4040; cyclooxygenase; erlotinib; nitric oxide; non-small-cell lung cancer; prostaglandin.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Nitro aspirin (NCX4040) induces apoptosis in PC3 metastatic prostate cancer cells via hydrogen peroxide (H2O2)-mediated oxidative stress.Free Radic Biol Med. 2019 Nov 1;143:494-509. doi: 10.1016/j.freeradbiomed.2019.08.025. Epub 2019 Aug 22. Free Radic Biol Med. 2019. PMID: 31446057 Free PMC article.
-
NCX 4040, a nitric oxide-donating aspirin, exerts anti-inflammatory effects through inhibition of I kappa B-alpha degradation in human monocytes.J Immunol. 2010 Feb 15;184(4):2140-7. doi: 10.4049/jimmunol.0903107. Epub 2010 Jan 11. J Immunol. 2010. PMID: 20065114
-
Gastroprotective and ulcer healing effects of nitric oxide-releasing non-steroidal anti-inflammatory drugs.Dig Liver Dis. 2000 Oct;32(7):583-94. doi: 10.1016/s1590-8658(00)80840-3. Dig Liver Dis. 2000. PMID: 11142556
-
Cox-2-selective inhibitors: the new super aspirins.Mol Pharmacol. 1999 Apr;55(4):625-31. Mol Pharmacol. 1999. PMID: 10101019 Review. No abstract available.
-
Therapeutic effects of nitric oxide-aspirin hybrid drugs.Expert Opin Ther Targets. 2006 Dec;10(6):911-22. doi: 10.1517/14728222.10.6.911. Expert Opin Ther Targets. 2006. PMID: 17105376 Review.
Cited by
-
Molecular Mechanisms of Cytotoxicity of NCX4040, the Non-Steroidal Anti-Inflammatory NO-Donor, in Human Ovarian Cancer Cells.Int J Mol Sci. 2022 Aug 3;23(15):8611. doi: 10.3390/ijms23158611. Int J Mol Sci. 2022. PMID: 35955744 Free PMC article.
References
-
- Santana-Davila R., Szabo A., Arce-Lara C., Williams C.D., Kelley M.J., Whittle J. Cisplatin versus carboplatin-based regimens for the treatment of patients with metastatic lung cancer. An analysis of Veterans Health Administration data. J. Thorac. Oncol. 2014;9:702–709. doi: 10.1097/JTO.0000000000000146. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

