Anti-invasive and Anti-tumor Effects of Dryopteris crassirhizoma Extract by Disturbing Actin Polymerization
- PMID: 31109222
- PMCID: PMC6537295
- DOI: 10.1177/1534735419851197
Anti-invasive and Anti-tumor Effects of Dryopteris crassirhizoma Extract by Disturbing Actin Polymerization
Abstract
Aim: To evaluate the anti-invasive effect of ethanol extracts of rhizome of Dryopteris crassirhizoma (EEDC) in matrix invasion and formation of functional invadopodia and to determine the anti-tumor effect of EEDC in a mouse model of mandibular invasion by gingival squamous cell carcinoma (SCC).
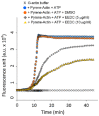
Methods: The rhizome of D crassirhizoma was extracted in ethanol. The anti-invasive effect of EEDC was analyzed with a Matrigel-coated transwell invasion and 3D culture system. Crucial factors related to the control of cancer cell invasion by EEDC were determined using a human protease array. Molecular evidence supporting the anti-invasive effect of EEDC in oral SCC (OSCC) cells used an invadopodia-mediated extracellular matrix (ECM) degradation; an in vivo athymic mouse model was also provided.
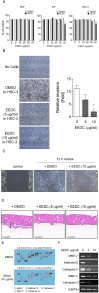
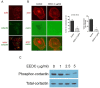
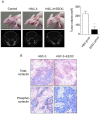
Results: EEDC treatment (10 µg/mL) suppressed transwell migration and invasion of HSC-3 OSCC cells without cytotoxicity. Decreased levels of matrix metalloprotease (MMP)-7, kalikrein 10, cathepsin V, MMP-2, and cathepsin D were also found in EEDC-treated HSC-3 cells based on human protease array. The anti-invasive effects of EEDC involved the suppression of invadopodia-mediated ECM degradation via inhibition of globular-actin elongation. The anti-invasive effect resulting from disturbance of functional invadopodia formation by EEDC was observed even at a low concentration of 5 µg/mL. The phosphorylation of cortactin involved in functional invadopodia formation was decreased at EEDC concentrations that inhibited invadopodia formation. The anti-tumor effect of EEDC was also observed in a mouse xenograft model. Administration of EEDC resulted in inhibition of tumor growth and progression.
Conclusions: EEDC represents a potential anti-invasive and anti-tumor agent in cancer control.
Keywords: actin polymerization; extracellular matrix; invadopodia; invasion; xenograft.
Conflict of interest statement
Figures
Similar articles
-
Epigallocatechin-3 gallate inhibits cancer invasion by repressing functional invadopodia formation in oral squamous cell carcinoma.Eur J Pharmacol. 2013 Sep 5;715(1-3):286-95. doi: 10.1016/j.ejphar.2013.05.008. Epub 2013 May 22. Eur J Pharmacol. 2013. PMID: 23707351
-
Invadopodia formation in oral squamous cell carcinoma: the role of epidermal growth factor receptor signalling.Arch Oral Biol. 2012 Apr;57(4):335-43. doi: 10.1016/j.archoralbio.2011.08.019. Epub 2011 Sep 13. Arch Oral Biol. 2012. PMID: 21920495
-
Dryopteris crassirhizoma has anti-cancer effects through both extrinsic and intrinsic apoptotic pathways and G0/G1 phase arrest in human prostate cancer cells.J Ethnopharmacol. 2010 Jul 20;130(2):248-54. doi: 10.1016/j.jep.2010.04.038. Epub 2010 May 8. J Ethnopharmacol. 2010. PMID: 20438825
-
Inhibition of invadopodia formation by diosgenin in tumor cells.Oncol Lett. 2020 Dec;20(6):283. doi: 10.3892/ol.2020.12148. Epub 2020 Sep 23. Oncol Lett. 2020. PMID: 33014161 Free PMC article. Review.
-
Nucleating actin for invasion.Nat Rev Cancer. 2011 Mar;11(3):177-87. doi: 10.1038/nrc3003. Epub 2011 Feb 10. Nat Rev Cancer. 2011. PMID: 21326322 Review.
Cited by
-
Comparison of Constituents and Antioxidant Activity of Above-Ground and Underground Parts of Dryopteris crassirhizoma Nakai Based on HS-SPME-GC-MS and UPLC/Q-TOF-MS.Molecules. 2022 Aug 5;27(15):4991. doi: 10.3390/molecules27154991. Molecules. 2022. PMID: 35956948 Free PMC article.
-
Anticoronaviral Activity of the Natural Phloroglucinols, Dryocrassin ABBA and Filixic Acid ABA from the Rhizome of Dryopteris crassirhizoma by Targeting the Main Protease of SARS-CoV-2.Pharmaceutics. 2022 Feb 8;14(2):376. doi: 10.3390/pharmaceutics14020376. Pharmaceutics. 2022. PMID: 35214108 Free PMC article.
-
Dryopteris juxtapostia Root and Shoot: Determination of Phytochemicals; Antioxidant, Anti-Inflammatory, and Hepatoprotective Effects; and Toxicity Assessment.Antioxidants (Basel). 2022 Aug 27;11(9):1670. doi: 10.3390/antiox11091670. Antioxidants (Basel). 2022. PMID: 36139744 Free PMC article.
-
Optimization of Ultrasonic-Assisted Extraction of α-Glucosidase Inhibitors from Dryopteris crassirhizoma Using Artificial Neural Network and Response Surface Methodology.Metabolites. 2023 Apr 13;13(4):557. doi: 10.3390/metabo13040557. Metabolites. 2023. PMID: 37110215 Free PMC article.
-
Inhibitory Effect and Mechanism of Dryocrassin ABBA Against Fusarium oxysporum.Int J Mol Sci. 2025 Feb 13;26(4):1573. doi: 10.3390/ijms26041573. Int J Mol Sci. 2025. PMID: 40004037 Free PMC article.
References
-
- Bhanot A, Sharma R, Noolvi MN. Natural sources as potential anti-cancer agents: a review. Int J Phytomedicine. 2011;3:9-26.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

