Sigma-2 receptor/TMEM97 agonist PB221 as an alternative drug for brain tumor
- PMID: 31109310
- PMCID: PMC6528305
- DOI: 10.1186/s12885-019-5700-7
Sigma-2 receptor/TMEM97 agonist PB221 as an alternative drug for brain tumor
Abstract
Background: There are limited effective drugs that can reach the brain to target brain tumors, in particular glioblastoma, which is one of the most difficult cancers to be cured from. Because the overexpression of the sigma-2 receptor is frequently reported in glioma clinical samples and associated with poor prognosis and malignancy, we herein studied the anti-tumor effect of the sigma-2 receptor agonist PB221 (4-cyclohexyl-1-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperidine) on an anaplastic astrocytoma tumor model based on previous encouraging results in pancreatic cancer and neuroblastoma SK-N-SH cells.
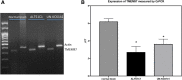
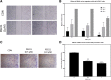
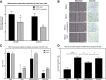
Methods: The expression of the sigma-2 receptor, transmembrane protein 97 (TMEM97), in ALTS1C1 and UN-KC6141 cell lines was measured by RT-PCR and quantitative RT-PCR. The binding of sigma-2 receptor fluorescent ligands PB385 (6-[5-[3-(4-cyclohexylpiperazin-1-yl)propyl]-5,6,7,8-tetrahydronaphthalen-5-yloxy]-N-(7-nitro-2,1,3-benzoxadiazol-4-yl)hexanamine) and NO1 (2-{6-[2-(3-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)propyl)-3,4-dihydroisoquinolin-1(2H)-one-5-yloxy]hexyl}-5-(dimethylamino)isoindoline-1,3-dione) was examined by flow cytometry and the fluorescent plate reader. The antitumor activity of PB221 was initially examined in the murine brain tumor cell line ALTS1C1 and then in the murine pancreatic cell line UN-KC6141. The potential therapeutic efficacy of PB221 for murine brain tumors was examined by in vitro migration and invasion assays and in vivo ectopic and orthotopic ALTS1C1 tumor models.
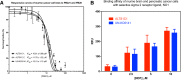
Results: The IC50 of PB221 for ALTS1C1 and UN-KC6141 cell lines was 10.61 ± 0.96 and 13.13 ± 1.15 μM, respectively. A low dose of PB221 (1 μM) significantly repressed the migration and invasion of ALTS1C1 cells, and a high dose of PB221 (20 μM) resulted in the apoptotic cell death of ALTS1C1 cells. These effects were reduced by the lipid antioxidant α-tocopherol, but not by the hydrophilic N-acetylcysteine, suggesting mitochondrial oxidative stress is involved. The in vivo study revealed that PB221 effectively retarded tumor growth to 36% of the control tumor volume in the ectopic intramuscular tumor model and increased the overall survival time by 20% (from 26 to 31 days) in the orthotopic intracerebral tumor model.
Conclusions: This study demonstrates that the sigma-2 receptor agonist PB221 has the potential to be an alternative chemotherapeutic drug for brain tumors with comparable side effects as the current standard-of-care drug, temozolomide.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Sigma-2 receptor agonist derivatives of 1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine (PB28) induce cell death via mitochondrial superoxide production and caspase activation in pancreatic cancer.BMC Cancer. 2017 Jan 13;17(1):51. doi: 10.1186/s12885-016-3040-4. BMC Cancer. 2017. PMID: 28086830 Free PMC article.
-
Investigation of σ receptors agonist/antagonist activity through N-(6-methoxytetralin-1-yl)- and N-(6-methoxynaphthalen-1-yl)alkyl derivatives of polymethylpiperidines.Bioorg Med Chem. 2013 Apr 1;21(7):1865-9. doi: 10.1016/j.bmc.2013.01.034. Epub 2013 Jan 29. Bioorg Med Chem. 2013. PMID: 23415062
-
Identification and characterization of MAM03055A: A novel bivalent sigma-2 receptor/TMEM97 ligand with cytotoxic activity.Eur J Pharmacol. 2021 Sep 5;906:174263. doi: 10.1016/j.ejphar.2021.174263. Epub 2021 Jun 16. Eur J Pharmacol. 2021. PMID: 34144027 Free PMC article.
-
Exploring the importance of piperazine N-atoms for sigma(2) receptor affinity and activity in a series of analogs of 1-cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine (PB28).J Med Chem. 2009 Dec 10;52(23):7817-28. doi: 10.1021/jm9007505. J Med Chem. 2009. PMID: 19842660
-
Contemporary murine models in preclinical astrocytoma drug development.Neuro Oncol. 2015 Jan;17(1):12-28. doi: 10.1093/neuonc/nou288. Epub 2014 Sep 21. Neuro Oncol. 2015. PMID: 25246428 Free PMC article. Review.
Cited by
-
Multi-Target Directed Ligands (MTDLs) Binding the σ1 Receptor as Promising Therapeutics: State of the Art and Perspectives.Int J Mol Sci. 2021 Jun 14;22(12):6359. doi: 10.3390/ijms22126359. Int J Mol Sci. 2021. PMID: 34198620 Free PMC article. Review.
-
Development of Fluorescent 4-[4-(3H-Spiro[isobenzofuran-1,4'-piperidin]-1'-yl)butyl]indolyl Derivatives as High-Affinity Probes to Enable the Study of σ Receptors via Fluorescence-Based Techniques.J Med Chem. 2023 Mar 23;66(6):3798-3817. doi: 10.1021/acs.jmedchem.2c01227. Epub 2023 Mar 15. J Med Chem. 2023. PMID: 36919956 Free PMC article.
-
The Sigma Enigma: A Narrative Review of Sigma Receptors.Cureus. 2023 Mar 4;15(3):e35756. doi: 10.7759/cureus.35756. eCollection 2023 Mar. Cureus. 2023. PMID: 37020478 Free PMC article. Review.
-
MRP1-Collateral Sensitizers as a Novel Therapeutic Approach in Resistant Cancer Therapy: An In Vitro and In Vivo Study in Lung Resistant Tumor.Int J Mol Sci. 2020 May 8;21(9):3333. doi: 10.3390/ijms21093333. Int J Mol Sci. 2020. PMID: 32397184 Free PMC article.
-
Exploring the RC-106 Chemical Space: Design and Synthesis of Novel (E)-1-(3-Arylbut-2-en-1-yl)-4-(Substituted) Piperazine Derivatives as Potential Anticancer Agents.Front Chem. 2020 Jun 30;8:495. doi: 10.3389/fchem.2020.00495. eCollection 2020. Front Chem. 2020. PMID: 32695745 Free PMC article.
References
-
- Anghel R, Gales LM, Bizu I, Ragan C, Mitrica R, Ion OG. Improved survival rate in patients with glioblastoma who underwent more than six cycles of temozolomide in a multimodal treatment scheme. J Clin Oncol. 2014;(15):32.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

