Effect of 6 months' flash glucose monitoring in adolescents and young adults with type 1 diabetes and suboptimal glycaemic control: managing diabetes in a 'flash' randomised controlled trial protocol
- PMID: 31109342
- PMCID: PMC6528266
- DOI: 10.1186/s12902-019-0378-z
Effect of 6 months' flash glucose monitoring in adolescents and young adults with type 1 diabetes and suboptimal glycaemic control: managing diabetes in a 'flash' randomised controlled trial protocol
Abstract
Background: Teenagers and young adults with type 1 diabetes (T1D) experience significant burden managing this serious chronic condition and glycaemic control is at its unhealthiest during this life stage. Flash glucose monitoring (FGM) is a new technology that reduces the burden of glucose monitoring by easily and discreetly displaying glucose information when an interstitial glucose sensor worn on the upper arm is scanned with a handheld reader, as opposed to traditional capillary glucose sampling by finger prick (otherwise known as self-monitored blood glucose, SMBG). The effectiveness of this technology and impacts of its long-term use in youth with pre-existing suboptimal glycaemic control are unknown. This study therefore aims to investigate the effectiveness of FGM in addition to standard care in young people with T1D.
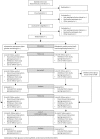
Methods: This is a two phase study programme including a multi-centre randomised, parallel-group study consisting of a 6-month comparison between SMBG and FGM, with an additional 6-month continuation phase. We will enrol adolescents with T1D aged 13-20 years (inclusive), with suboptimal glycaemic control (mean glycated haemoglobin (HbA1c) in past 6 months ≥75 mmol/mol [≥9%]). Participants will be randomly allocated (1:1) to FGM (FreeStyle Libre; intervention group) or to continue SMBG with capillary blood glucose testing (usual care group). All participants will continue other aspects of standard care with the study only providing the FreeStyle Libre. At 6 months, the control group will cross over to the intervention. The primary outcome is the between group difference in changes in HbA1c at 6 months. Additional outcomes include a range of psychosocial and health economic measures as well as FGM acceptability.
Discussion: >If improvements are found, this will further encourage steps towards integrating FGM into regular diabetes care for youth with unhealthy glycaemic control, with the expectation it will reduce daily diabetes management burden and improve short- and long-term health outcomes in this high-risk group.
Trial registration: This trial was registered with the Australian New Zealand Clinical Trials Registry on 5 March 2018 ( ACTRN12618000320257p ) and the World Health Organization International Clinical Trials Registry Platform (Universal Trial Number U1111-1205-5784).
Keywords: Adolescents; Flash glucose monitoring; FreeStyle libre; Glucose monitoring; Glycaemic control; Intermittent continuous glucose monitoring; Self-monitoring of blood glucose; Type 1 diabetes; Young adults.
Conflict of interest statement
The authors declare that they have no competing interests.
References
-
- Mayer-Davis EJ, Kahkoska AR, Jefferies C, Dabelea D, Balde N, Gon CX, Aschner P, Craig ME. ISPAD clinical practice consensus guidelines 2018: definition, epidemiology and classification of diabetes in children and adolescents. Pediatr Diabetes. 2018;19(Supp. 27):7–19. doi: 10.1111/pedi.12773. - DOI - PMC - PubMed
-
- Ogurtsova K, da Rocha Fernandes J, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017. 10.1016/j.diabres.2017.03.024. - PubMed
-
- Campbell-Stokes P, Taylor B. Prospective incidence study of diabetes mellitus in New Zealand children aged 0 to 14 years. Diabetologia. 2005. 10.1007/s00125-005-1697-3. - PubMed
-
- Fox DA, Islam N, Sutherland J, et al. Type 1 diabetes incidence and prevalence trends in a cohort of Canadian children and youth. Pediatr Diabetes. 2018. 10.1111/pedi.12566. - PubMed
-
- Bendas A, Rothe U, Kiess W, Kapellen TM, Stange T, Manuwald U, Salzsieder E, Holl RW, Schoffer O, Stahl-Pehe A. Trends in incidence rates during 1999-2008 and prevalence in 2008 of childhood type 1 diabetes mellitus in Germany–model-based National Estimates. PLoS One. 2015;10(7):e0123716. doi: 10.1371/journal.pone.0132716. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous