Proteoglycans involved in bidirectional communication between mast cells and hippocampal neurons
- PMID: 31109355
- PMCID: PMC6528191
- DOI: 10.1186/s12974-019-1504-6
Proteoglycans involved in bidirectional communication between mast cells and hippocampal neurons
Abstract
Background: Mast cells (MCs) in the brain can respond to environmental cues and relay signals to neurons that may directly influence neuronal electrical activity, calcium signaling, and neurotransmission. MCs also express receptors for neurotransmitters and consequently can be activated by them. Here, we developed a coculture model of peritoneal MCs, incubated together with dissociated hippocampal neurons for the study of cellular mechanisms involved in the mast cell-neuron interactions.
Methods: Calcium imaging was used to simultaneously record changes in intracellular calcium [Ca2+]i in neurons and MCs. To provide insight into the contribution of MCs on neurotransmitter release in rat hippocampal neurons, we used analysis of FM dye release, evoked by a cocktail of mediators from MCs stimulated by heat.
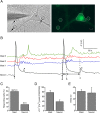
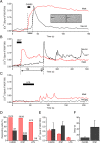
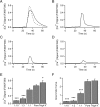
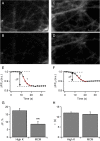
Results: Bidirectional communication is set up between MCs and hippocampal neurons. Neuronal depolarization caused intracellular calcium [Ca2+]i oscillations in MCs that produced a quick response in neurons. Furthermore, activation of MCs with antigen or the secretagogue compound 48/80 also resulted in a neuronal [Ca2+]i response. Moreover, local application onto neurons of the MC mediator cocktail elicited Ca2+ transients and a synaptic release associated with FM dye destaining. Neuronal response was partially blocked by D-APV, a N-methyl-D-aspartate receptor (NMDAR) antagonist, and was inhibited when the cocktail was pre-digested with chondroitinase ABC, which induces enzymatic removal of proteoglycans of chondroitin sulfate (CS).
Conclusions: MC-hippocampal neuron interaction affects neuronal [Ca2+]i and exocytosis signaling through a NMDAR-dependent mechanism.
Keywords: Ca2+ imaging; NMDA receptors; exocytosis; hippocampal neurotransmission; neuro-immune; proteoglycans.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Astrocytes in 17beta-estradiol treated mixed hippocampal cultures show attenuated calcium response to neuronal activity.Glia. 2006 Jun;53(8):817-26. doi: 10.1002/glia.20341. Glia. 2006. PMID: 16565986
-
Mast Cell Changes the Phenotype of Microglia via Histamine and ATP.Cell Physiol Biochem. 2021 Jan 15;55(1):17-32. doi: 10.33594/000000324. Cell Physiol Biochem. 2021. PMID: 33443845
-
Astrocytic control of synaptic NMDA receptors.J Physiol. 2007 Jun 15;581(Pt 3):1057-81. doi: 10.1113/jphysiol.2007.130377. Epub 2007 Apr 5. J Physiol. 2007. PMID: 17412766 Free PMC article.
-
Remodeling of Intracellular Ca2+ Homeostasis in Rat Hippocampal Neurons Aged In Vitro.Int J Mol Sci. 2020 Feb 24;21(4):1549. doi: 10.3390/ijms21041549. Int J Mol Sci. 2020. PMID: 32102482 Free PMC article. Review.
-
MRGPRX2 signals its importance in cutaneous mast cell biology: Does MRGPRX2 connect mast cells and atopic dermatitis?Exp Dermatol. 2020 Nov;29(11):1104-1111. doi: 10.1111/exd.14182. Epub 2020 Sep 17. Exp Dermatol. 2020. PMID: 32866307 Review.
Cited by
-
Paraventricular Mast Cell-Derived Histamine Activates CRH Neurons to Mediate Adult Visceral Hypersensitivity Induced by Neonatal Maternal Separation.Brain Sci. 2023 Nov 16;13(11):1595. doi: 10.3390/brainsci13111595. Brain Sci. 2023. PMID: 38002554 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

