Prognostic risk score for patients with relapsed or refractory chronic lymphocytic leukaemia treated with targeted therapies or chemoimmunotherapy: a retrospective, pooled cohort study with external validations
- PMID: 31109827
- PMCID: PMC6620111
- DOI: 10.1016/S2352-3026(19)30085-7
Prognostic risk score for patients with relapsed or refractory chronic lymphocytic leukaemia treated with targeted therapies or chemoimmunotherapy: a retrospective, pooled cohort study with external validations
Erratum in
-
Correction to Lancet Haematol 2019; 6: e366-74.Lancet Haematol. 2019 Jul;6(7):e348. doi: 10.1016/S2352-3026(19)30116-4. Lancet Haematol. 2019. PMID: 31244475 No abstract available.
Abstract
Background: Clinically validated prognostic models for overall survival do not exist for patients with relapsed or refractory chronic lymphocytic leukaemia (CLL) who are on targeted therapies. We aimed to create a prognostic model to identify high-risk individuals who do not achieve a good outcome with available targeted therapies.
Methods: In this retrospective, pooled cohort study, 2475 patients with CLL treated between June 22, 2012, and Sept 23, 2015, in six randomised trials of ibrutinib, idelalisib, and venetoclax, or at the Mayo Clinic CLL Database (MCCD) were included. Eligible patients had CLL, were previously treated, were aged 18 years or older, had ECOG performance status 0-1, and required further treatment as per the international workshop on CLL 2008 criteria. There was heterogeneity in other eligibility criteria. We evaluated 28 candidate factors known to affect the overall survival of these patients and applied univariate and multivariate analyses to derive the risk score in a training dataset (n=727) of patients treated with ibrutinib or chemoimmunotherapy. We validated the score in an internal-validation dataset (n=242) of patients treated with ibrutinib or chemoimmunotherapy and three external-validation datasets (idelalisib or chemoimmunotherapy dataset, n=897; venetoclax or chemoimmunotherapy dataset, n=389; and the MCCD [including patients treated with heterogeneous therapies], n=220), applying C-statistics as a measure of discrimination.
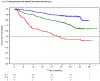
Findings: The derived model consisted of four factors (one point each; serum β2-microglobulin ≥5 mg/dL, lactate dehydrogenase >upper limit of normal, haemoglobin <110 g/L for women or <120 g/L for men, and time from initiation of last therapy <24 months), separating patients into low (score 0-1), intermediate (score 2-3), and high risk (score 4) groups. The risk score was prognostic for overall survival in the training dataset (CS=0·74, 95% CI 0·60-0·85, log-rank p<0·0001), and in the internal-validation (CS=0·79, 0·56-0·97, log-rank p=0·0003), and all three external-validation cohorts (idelalisib or chemoimmunotherapy: CS=0·71, 0·59-0·81, log-rank p<0·0001; venetoclax or chemoimmunotherapy: CS =0·76, 0·66-0·85, log-rank p=0·014; MCCD cohort: CS=0·61, 0·56-0·66), log-rank p<0·0001). The risk score is available on Calculate by QxMD.
Interpretation: We present the first validated risk score to predict overall survival in patients with relapsed or refractory CLL treated with targeted therapy. The model is applicable to patients treated with all currently approved targeted therapies (ibrutinib, idelalisib, and venetoclax) and chemoimmunotherapy. This tool allows the identification of a well defined cohort of previously treated patients with CLL who are at high risk of death, and could be used in future prospective trials to test therapeutic options for these patients with an unmet clinical need.
Funding: Lymphoma Research Foundation, Lymphoma Research Fund (Andrew D Zelenetz), and National Institutes of Health/National Cancer Institute.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures




Comment in
-
On the BALL to spot the best score able to predict overall survival in relapsed or refractory CLL.Lancet Haematol. 2019 Jul;6(7):e343-e344. doi: 10.1016/S2352-3026(19)30091-2. Epub 2019 May 17. Lancet Haematol. 2019. PMID: 31109826 No abstract available.
References
-
- Rai KR, Sawitsky A, Cronkite E, Chanana A, Levy R, Pasternack B. Clinical staging of chronic lymphocytic leukemia. Blood. Blood 1975; 46: 219–34. - PubMed
-
- Binet JL, Auquier A, Dighiero G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer 1981; 48: 198–206. - PubMed
-
- Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig VH Genes Are Associated With a More Aggressive Form of Chronic Lymphocytic Leukemia. Blood 1999; 94 http://www.bloodjournal.org/content/94/6/1848.short (accessed March 29, 2017). - PubMed
-
- Stilgenbauer S, Schnaiter A, Paschka P, et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood 2014; 123: 3247–54. - PubMed
-
- Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 2000; 343: 1910–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

