Amino Acid Biosynthetic Pathways Are Required for Klebsiella pneumoniae Growth in Immunocompromised Lungs and Are Druggable Targets during Infection
- PMID: 31109974
- PMCID: PMC6658747
- DOI: 10.1128/AAC.02674-18
Amino Acid Biosynthetic Pathways Are Required for Klebsiella pneumoniae Growth in Immunocompromised Lungs and Are Druggable Targets during Infection
Abstract
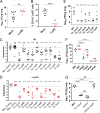
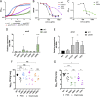
The emergence of multidrug-resistant Klebsiella pneumoniae has rendered a large array of infections difficult to treat. In a high-throughput genetic screen of factors required for K. pneumoniae survival in the lung, amino acid biosynthesis genes were critical for infection in both immunosuppressed and wild-type (WT) mice. The limited pool of amino acids in the lung did not change during infection and was insufficient for K. pneumoniae to overcome attenuating mutations in aroA, hisA, leuA, leuB, serA, serB, trpE, and tyrA in WT and immunosuppressed mice. Deletion of aroA, which encodes 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase class I, resulted in the most severe attenuation. Treatment with the EPSP synthase-specific competitive inhibitor glyphosate decreased K. pneumoniae growth in the lungs. K. pneumoniae expressing two previously identified glyphosate-resistant mutations in EPSP synthase had significant colonization defects in lung infection. Selection and characterization of six spontaneously glyphosate-resistant mutants in K. pneumoniae yielded no mutations in aroA Strikingly, glyphosate treatment of mice lowered the bacterial burden of two of three spontaneous glyphosate-resistant mutants and further lowered the burden of the less-attenuated EPSP synthase catalytic mutant. Of 39 clinical isolate strains, 9 were resistant to glyphosate at levels comparable to those of selected resistant strains, and none appeared to be more highly resistant. These findings demonstrate amino acid biosynthetic pathways essential for K. pneumoniae infection are promising novel therapeutic targets.
Keywords: Klebsiella pneumoniae; amino acid biosynthesis; aroA; glyphosate; multidrug resistant; neutropenic.
Copyright © 2019 American Society for Microbiology.
Figures



References
-
- Davis GS, Waits K, Nordstrom L, Weaver B, Aziz M, Gauld L, Grande H, Bigler R, Horwinski J, Porter S, Stegger M, Johnson JR, Liu CM, Price LB. 2015. Intermingled Klebsiella pneumoniae populations between retail meats and human urinary tract infections. Clin Infect Dis 61:892–899. doi: 10.1093/cid/civ428. - DOI - PMC - PubMed
-
- Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, Alberti S, Bush K, Tenover FC. 2001. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 45:1151–1161. doi: 10.1128/AAC.45.4.1151-1161.2001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

