Sodium Zirconium Cyclosilicate among Individuals with Hyperkalemia: A 12-Month Phase 3 Study
- PMID: 31110051
- PMCID: PMC6556727
- DOI: 10.2215/CJN.12651018
Sodium Zirconium Cyclosilicate among Individuals with Hyperkalemia: A 12-Month Phase 3 Study
Abstract
Background and objectives: Oral sodium zirconium cyclosilicate (formerly ZS-9) binds and removes potassium via the gastrointestinal tract. Sodium zirconium cyclosilicate-associated restoration and maintenance of normokalemia and adverse events were evaluated in a two-part, open label, phase 3 trial.
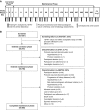
Design, setting, participants, & measurements: In the correction phase, adult outpatients with plasma potassium ≥5.1 mmol/L (i-STAT Point-of-Care) received sodium zirconium cyclosilicate 10 g three times daily for 24-72 hours until normokalemic (potassium =3.5-5.0 mmol/L). Qualifying participants entered the ≤12-month maintenance phase and received sodium zirconium cyclosilicate 5 g once daily titrated to maintain normokalemia without dietary or medication restrictions. Prespecified primary end points were restoration of normal serum potassium values (3.5-5.0 mmol/L) during the correction phase and maintenance of serum potassium ≤5.1 mmol/L during the maintenance phase. Adverse events were assessed throughout.
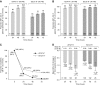
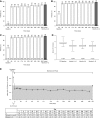
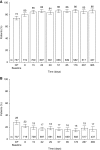
Results: Of 751 participants, 746 (99%) achieved normokalemia during the correction phase (mean serum potassium =4.8 mmol/L; 95% confidence interval, 4.7 to 4.8) and entered the maintenance phase; 466 (63%) participants completed the 12-month trial. Participants were predominantly white, men, and age ≥65 years old; 74% had an eGFR<60 ml/min per 1.73 m2, and 65% used renin-angiotensin-aldosterone system inhibitors. Mean time on sodium zirconium cyclosilicate was 286 days. Mean daily sodium zirconium cyclosilicate dose was 7.2 g (SD=2.6). Over months 3-12, mean serum potassium was 4.7 mmol/L (95% confidence interval, 4.6 to 4.7); mean serum potassium values ≤5.1 and ≤5.5 mmol/L were achieved by 88% and 99% of participants, respectively. Of 483 renin-angiotensin-aldosterone system inhibitor users at baseline, 87% continued or had their dose increased; 11% discontinued. Among 263 renin-angiotensin-aldosterone system inhibitor-naïve participants, 14% initiated renin-angiotensin-aldosterone system inhibitor therapy. Overall, 489 (66%) participants experienced adverse events during the maintenance phase, and 22% experienced a serious adverse event. Of eight (1%) deaths, none were considered related to sodium zirconium cyclosilicate. Nine (1%) and 34 (5%) participants experienced serum potassium <3.0 and 3.0-3.4 mmol/L, respectively.
Conclusions: After achieving normokalemia, individualized once daily sodium zirconium cyclosilicate was associated with maintenance of normokalemia without substantial renin-angiotensin-aldosterone system inhibitor changes for ≤12 months.
Keywords: Confidence Intervals; EGFR protein, human; Gastrointestinal Tract; Outpatients; Plasma; Point-of-Care Systems; Potassium; Receptor, Epidermal Growth Factor; Sodium; Zirconium; antibiotic K 4; chronic kidney disease; hyperkalemia; renin angiotensin system; sodium zirconium cyclosilicate (SZC).
Copyright © 2019 by the American Society of Nephrology.
Figures

Comment in
-
Therapeutic Potential of Newer Drugs for Treating Hyperkalemia.Clin J Am Soc Nephrol. 2019 Jun 7;14(6):787-788. doi: 10.2215/CJN.04750419. Epub 2019 May 20. Clin J Am Soc Nephrol. 2019. PMID: 31110052 Free PMC article. No abstract available.
References
-
- Kovesdy CP: Management of hyperkalemia: An update for the internist. Am J Med 128: 1281–1287, 2015 - PubMed
-
- McCullough PA, Costanzo MR, Silver M, Spinowitz B, Zhang J, Lepor NE: Novel agents for the prevention and management of hyperkalemia. Rev Cardiovasc Med 16: 140–155, 2015 - PubMed
-
- Kovesdy CP, Appel LJ, Grams ME, Gutekunst L, McCullough PA, Palmer BF, Pitt B, Sica DA, Townsend RR: Potassium homeostasis in health and disease: A scientific workshop cosponsored by the National Kidney Foundation and the American Society of Hypertension. J Am Soc Hypertens 11: 783–800, 2017 - PubMed
-
- McCullough PA, Beaver TM, Bennett-Guerrero E, Emmett M, Fonarow GC, Goyal A, Herzog CA, Kosiborod M, Palmer BF: Acute and chronic cardiovascular effects of hyperkalemia: New insights into prevention and clinical management. Rev Cardiovasc Med 15: 11–23, 2014 - PubMed
-
- McCullough PA, Rangaswami J: Real or perceived: Hyperkalemia is a major deterrent for renin-angiotensin aldosterone system inhibition in heart failure. Nephron 138: 173–175, 2018 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

