Cerebellar Stellate Cell Excitability Is Coordinated by Shifts in the Gating Behavior of Voltage-Gated Na+ and A-Type K+ Channels
- PMID: 31110133
- PMCID: PMC6553571
- DOI: 10.1523/ENEURO.0126-19.2019
Cerebellar Stellate Cell Excitability Is Coordinated by Shifts in the Gating Behavior of Voltage-Gated Na+ and A-Type K+ Channels
Abstract
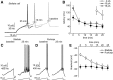
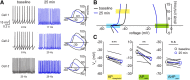
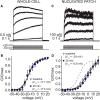
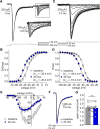
Neuronal excitability in the vertebrate brain is governed by the coordinated activity of both ligand- and voltage-gated ion channels. In the cerebellum, spontaneous action potential (AP) firing of inhibitory stellate cells (SCs) is variable, typically operating within the 5- to 30-Hz frequency range. AP frequency is shaped by the activity of somatodendritic A-type K+ channels and the inhibitory effect of GABAergic transmission. An added complication, however, is that whole-cell recording from SCs induces a time-dependent and sustained increase in membrane excitability making it difficult to define the full range of firing rates. Here, we show that whole-cell recording in cerebellar SCs of both male and female mice augments firing rates by reducing the membrane potential at which APs are initiated. AP threshold is lowered due to a hyperpolarizing shift in the gating behavior of voltage-gated Na+ channels. Whole-cell recording also elicits a hyperpolarizing shift in the gating behavior of A-type K+ channels which contributes to increased firing rates. Hodgkin-Huxley modeling and pharmacological experiments reveal that gating shifts in A-type K+ channel activity do not impact AP threshold, but rather promote channel inactivation which removes restraint on the upper limit of firing rates. Taken together, our work reveals an unappreciated impact of voltage-gated Na+ channels that work in coordination with A-type K+ channels to regulate the firing frequency of cerebellar SCs.
Keywords: A-type potassium channel; action potential; cerebellum; computational modeling; sodium channel; stellate cell.
Copyright © 2019 Alexander et al.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
