Predictor of Early Remission of Diabetic Macular Edema under As-Needed Intravitreal Ranibizumab
- PMID: 31110273
- PMCID: PMC6527559
- DOI: 10.1038/s41598-019-44078-6
Predictor of Early Remission of Diabetic Macular Edema under As-Needed Intravitreal Ranibizumab
Abstract
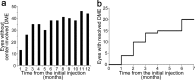
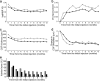
The early remission of diabetic macular edema (DME) often occurs in eyes treated with anti-vascular endothelial growth factor (VEGF) treatment. We retrospectively reviewed and characterized eyes with early remission of DME at six months in 80 eyes under pro re nata (PRN) intravitreal ranibizumab (IVR) injections. The number of eyes without center-involved DME gradually increased and 14 and 20 eyes achieved remission of DME at 3 or 6 months, respectively, under the PRN regimen following three monthly loading doses. In particular, eyes with early remission at 6 months had smaller CSF thickness than those without the remission before and after the treatment except at the 1-month visit (P < 0.05); however, the changes in CSF thickness did not differ between them. VA and its changes were not different between eyes with and without remission. Multivariate analysis revealed that smaller CSF thickness at baseline predicted the early remission of DME under PRN IVR injections (odds ratio, 0.989; 95% confidence interval, 0.982-0.997; P = 0.008). These data elucidate the clinical characteristics of early remission of DME under PRN IVR injections and suggest that smaller CSF thickness at baseline is a novel predictor of early remission under PRN IVR injections for DME.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Relation between macular morphology and treatment frequency during twelve months with ranibizumab for diabetic macular edema.PLoS One. 2017 Apr 13;12(4):e0175809. doi: 10.1371/journal.pone.0175809. eCollection 2017. PLoS One. 2017. PMID: 28407012 Free PMC article.
-
Anatomical and functional responses in eyes with diabetic macular edema treated with "1 + PRN" ranibizumab: one-year outcomes in population of mainland China.BMC Ophthalmol. 2020 Jun 15;20(1):229. doi: 10.1186/s12886-020-01510-0. BMC Ophthalmol. 2020. PMID: 32539744 Free PMC article.
-
Effect of Adding Dexamethasone to Continued Ranibizumab Treatment in Patients With Persistent Diabetic Macular Edema: A DRCR Network Phase 2 Randomized Clinical Trial.JAMA Ophthalmol. 2018 Jan 1;136(1):29-38. doi: 10.1001/jamaophthalmol.2017.4914. JAMA Ophthalmol. 2018. PMID: 29127949 Free PMC article. Clinical Trial.
-
Intravitreal Ranibizumab Alone or in Combination with Calcium Dobesilate for the Treatment of Diabetic Macular Edema in Nonproliferative Diabetic Retinopathy Patients: 12-Month Outcomes of a Retrospective Study.Int J Clin Pract. 2022 Oct 20;2022:6725225. doi: 10.1155/2022/6725225. eCollection 2022. Int J Clin Pract. 2022. PMID: 36340967 Free PMC article. Review.
-
Recent clinically relevant highlights from the Diabetic Retinopathy Clinical Research Network.Curr Opin Ophthalmol. 2018 May;29(3):199-205. doi: 10.1097/ICU.0000000000000472. Curr Opin Ophthalmol. 2018. PMID: 29528861 Review.
Cited by
-
Vitrectomized versus non-vitrectomized eyes in diabetic macular edema response to ranibizumab-retinal layers thickness as prognostic biomarkers.Sci Rep. 2021 Nov 29;11(1):23055. doi: 10.1038/s41598-021-02532-4. Sci Rep. 2021. PMID: 34845300 Free PMC article.
-
Foveal Eversion: A Possible Biomarker of Persistent Diabetic Macular Edema.Ophthalmol Ther. 2021 Mar;10(1):115-126. doi: 10.1007/s40123-020-00324-z. Epub 2021 Jan 9. Ophthalmol Ther. 2021. PMID: 33420954 Free PMC article.
-
Hyperreflective Foci and Subretinal Fluid Are Potential Imaging Biomarkers to Evaluate Anti-VEGF Effect in Diabetic Macular Edema.Front Physiol. 2021 Dec 23;12:791442. doi: 10.3389/fphys.2021.791442. eCollection 2021. Front Physiol. 2021. PMID: 35002773 Free PMC article.
-
Management of Diabetic Macular Edema: Guidelines from the Emirates Society of Ophthalmology.Ophthalmol Ther. 2022 Oct;11(5):1937-1950. doi: 10.1007/s40123-022-00547-2. Epub 2022 Jul 27. Ophthalmol Ther. 2022. PMID: 35896888 Free PMC article.
-
The Area and Number of Intraretinal Cystoid Spaces Predict the Visual Outcome after Ranibizumab Monotherapy in Diabetic Macular Edema.J Clin Med. 2020 May 8;9(5):1391. doi: 10.3390/jcm9051391. J Clin Med. 2020. PMID: 32397232 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

