Delivery of miRNA-29b Using R9-LK15, a Novel Cell-Penetrating Peptide, Promotes Osteogenic Differentiation of Bone Mesenchymal Stem Cells
- PMID: 31111046
- PMCID: PMC6487134
- DOI: 10.1155/2019/3032158
Delivery of miRNA-29b Using R9-LK15, a Novel Cell-Penetrating Peptide, Promotes Osteogenic Differentiation of Bone Mesenchymal Stem Cells
Abstract
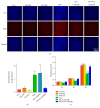
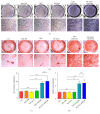
Delivery of osteogenesis-promoting microRNAs (miRNAs) is a promising approach to enhance bone regeneration. In this study, we generated nanocomplexes comprising the novel cell-penetrating peptide R9-LK15 and miR-29b and investigated their effects on osteogenic differentiation of bone mesenchymal stem cells (BMSCs). R9-LK15/miR-29b nanocomplexes were prepared and characterized. The transfection efficiency, cell viability, and osteogenic differentiation were investigated. The results showed that R9-LK15 maintained the stability of miR-29b in serum for up to 24 h. Moreover, R9-LK15 efficiently delivered miR-29b into BMSCs; the transfection efficiency was ~10-fold higher than that achieved using Lipofectamine 2000. The Cell Counting Kit-8 assay showed that R9-LK15 and R9-LK15/miR-29b nanocomplexes had negligible cytotoxic effects on BMSCs. Delivery of R9-LK15/miR-29b nanocomplexes promoted osteogenic differentiation of BMSCs and extracellular matrix mineralization by upregulating alkaline phosphatase expression and downregulating histone deacetylase-4 expression. In general, we developed a novel miRNA delivery system that has a high transfection efficiency and promotes osteogenic differentiation.
Figures




References
-
- Curtin C. M., Castaño I. M., O'Brien F. J. Scaffold-based microRNA therapies in regenerative medicine and cancer. Advanced Healthcare Materials. 2018;7(1) - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

