Intracellular Pathogens: Host Immunity and Microbial Persistence Strategies
- PMID: 31111075
- PMCID: PMC6487120
- DOI: 10.1155/2019/1356540
Intracellular Pathogens: Host Immunity and Microbial Persistence Strategies
Abstract
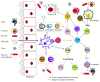
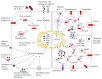
Infectious diseases caused by pathogens including viruses, bacteria, fungi, and parasites are ranked as the second leading cause of death worldwide by the World Health Organization. Despite tremendous improvements in global public health since 1950, a number of challenges remain to either prevent or eradicate infectious diseases. Many pathogens can cause acute infections that are effectively cleared by the host immunity, but a subcategory of these pathogens called "intracellular pathogens" can establish persistent and sometimes lifelong infections. Several of these intracellular pathogens manage to evade the host immune monitoring and cause disease by replicating inside the host cells. These pathogens have evolved diverse immune escape strategies and overcome immune responses by residing and multiplying inside host immune cells, primarily macrophages. While these intracellular pathogens that cause persistent infections are phylogenetically diverse and engage in diverse immune evasion and persistence strategies, they share common pathogen type-specific mechanisms during host-pathogen interaction inside host cells. Likewise, the host immune system is also equipped with a diverse range of effector functions to fight against the establishment of pathogen persistence and subsequent host damage. This article provides an overview of the immune effector functions used by the host to counter pathogens and various persistence strategies used by intracellular pathogens to counter host immunity, which enables their extended period of colonization in the host. The improved understanding of persistent intracellular pathogen-derived infections will contribute to develop improved disease diagnostics, therapeutics, and prophylactics.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

