Left atrial effective conducting size predicts atrial fibrillation vulnerability in persistent but not paroxysmal atrial fibrillation
- PMID: 31111557
- PMCID: PMC6746623
- DOI: 10.1111/jce.13990
Left atrial effective conducting size predicts atrial fibrillation vulnerability in persistent but not paroxysmal atrial fibrillation
Abstract
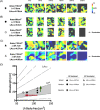
Background: The multiple wavelets and functional re-entry hypotheses are mechanistic theories to explain atrial fibrillation (AF). If valid, a chamber's ability to support AF should depend upon the left atrial size, conduction velocity (CV), and refractoriness. Measurement of these parameters could provide a new therapeutic target for AF. We investigated the relationship between left atrial effective conducting size (LAECS ), a function of area, CV and refractoriness, and AF vulnerability in patients undergoing AF ablation.
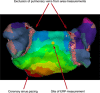
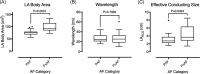
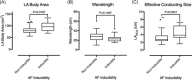
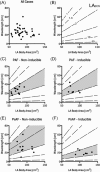
Methods and results: Activation mapping was performed in patients with paroxysmal (n = 21) and persistent AF (n = 18) undergoing pulmonary vein isolation. Parameters used for calculating LAECS were: (a) left atrial body area (A); (b) effective refractory period (ERP); and (c) total activation time (T). Global CV was estimated as . Effective atrial conducting size was calculated as . Post ablation, AF inducibility testing was performed. The critical LAECS required for multiple wavelet termination was determined from computational modeling. LAECS was greater in patients with persistent vs paroxysmal AF (4.4 ± 2.0 cm vs 3.2 ± 1.4 cm; P = .049). AF was inducible in 14/39 patients. LAECS was greater in AF-inducible patients (4.4 ± 1.8 cm vs 3.3 ± 1.7 cm; P = .035, respectively). The difference in LAECS between inducible and noninducible patients was significant in patients with persistent (P = .0046) but not paroxysmal AF (P = .6359). Computational modeling confirmed that LAECS > 4 cm was required for continuation of AF.
Conclusions: LAECS measured post ablation was associated with AF inducibility in patients with persistent, but not paroxysmal AF. These data support a role for this method in electrical substrate assessment in AF patients.
Keywords: atrial fibrillation vulnerability; conduction velocity; left atrial effective conducting size; refractoriness.
© 2019 The Authors Journal of Cardiovascular ElectrophysiologyPublished by Wiley Periodicals, Inc.
Conflict of interest statement
Professor O’Neill has received research support and honoraria from Biosense Webster and has received consultation fees from Medtronic, Biosense Webster, St. Jude/Abbott and Siemens. Dr. Niederer has received research support from St. Jude/Abbott, Boston Scientific, Roche, Pfizer and Siemens.
Figures



References
-
- Moe GK, Abildskov JA. Atrial fibrillation as a self‐sustaining arrhythmia independent of focal discharge. Am Heart J. 1959;58:59‐70. - PubMed
-
- Allessie MA, Lammers W, Bonke F, Hollen J. Experimental evaluation of Moe's multiple wavelet hypothesis of atrial fibrillation In: Zipes DP, Jalife J, eds. Cardiac Electrophysiology and Arrhythmias. Orlando, FL: Grune & Stratton, Inc; 1985:265‐276.
-
- Lee G, Kumar S, Teh A, et al. Epicardial wave mapping in human long‐lasting persistent atrial fibrillation: transient rotational circuits, complex wavefronts, and disorganized activity. Eur Heart J. 2014;35:86‐97. - PubMed
-
- Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The “leading circle” concept: a new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ Res. 1977;41:9‐18. - PubMed
Publication types
MeSH terms
Grants and funding
- WT 203148/Z/16/Z/BHF_/British Heart Foundation/United Kingdom
- FS/18/27/33543/BHF_/British Heart Foundation/United Kingdom
- PG/13/37/30280/BHF_/British Heart Foundation/United Kingdom
- MR/N001877/1/Medical Research Council UK/International
- MR/N001877/1Disclosures: None./Medical Research Council UK Clinical Research Training Fellowship/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

