Dihydromyricetin protects HUVECs of oxidative damage induced by sodium nitroprusside through activating PI3K/Akt/FoxO3a signalling pathway
- PMID: 31111658
- PMCID: PMC6584490
- DOI: 10.1111/jcmm.14406
Dihydromyricetin protects HUVECs of oxidative damage induced by sodium nitroprusside through activating PI3K/Akt/FoxO3a signalling pathway
Abstract
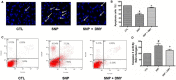
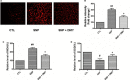
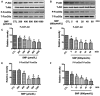
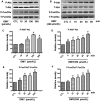
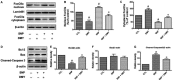
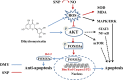
The damage of vascular endothelial cells induced by oxidative stress plays an important role in the pathogenesis of atherosclerosis. Dihydromyricetin (DMY) is considered as a natural antioxidant. However, the mechanism of DMY on endothelial cell injury induced by oxidative stress remains unclear. In this study, we found that DMY could reduce the oxidative damage of HUVECs induced by sodium nitroprusside (SNP), HUVECs pre-treated with DMY suppressed SNP-induced apoptosis by reduced ROS overproduction of intracellular, decreased MDA level and elevated the superoxide dismutase activity. Meanwhile, we found that DMY could promote the expression of phosphorylated FoxO3a and Akt, and affect the nuclear localization of FoxO3a, when treated with the PI3K inhibitor LY294002, the effect of DMY was blocked. These data suggest that DMY protects HUVECs from oxidative stress by activating PI3K/Akt/FoxO3a signalling pathway. Therefore, DMY may have great therapeutic potential as a new drug for atherosclerosis.
Keywords: apoptosis; atherosclerosis; dihydromyricetin; oxidative stress; sodium nitroprusside.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
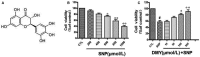
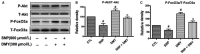

References
-
- Mozaffarian . Heart disease and stroke statistics‐2015 update: A report from the American heart association. Circulation. 2015; 131: E535. - PubMed
-
- Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852‐1866. - PubMed
-
- Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Antioxidative stress‐associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591‐3597. - PubMed
-
- Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73:411‐418. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

