The Bacillus cereus Group: Bacillus Species with Pathogenic Potential
- PMID: 31111815
- PMCID: PMC6530592
- DOI: 10.1128/microbiolspec.GPP3-0032-2018
The Bacillus cereus Group: Bacillus Species with Pathogenic Potential
Abstract
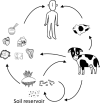
The Bacillus cereus group includes several Bacillus species with closely related phylogeny. The most well-studied members of the group, B. anthracis, B. cereus, and B. thuringiensis, are known for their pathogenic potential. Here, we present the historical rationale for speciation and discuss shared and unique features of these bacteria. Aspects of cell morphology and physiology, and genome sequence similarity and gene synteny support close evolutionary relationships for these three species. For many strains, distinct differences in virulence factor synthesis provide facile means for species assignment. B. anthracis is the causative agent of anthrax. Some B. cereus strains are commonly recognized as food poisoning agents, but strains can also cause localized wound and eye infections as well as systemic disease. Certain B. thuringiensis strains are entomopathogens and have been commercialized for use as biopesticides, while some strains have been reported to cause infection in immunocompromised individuals. In this article we compare and contrast B. anthracis, B. cereus, and B. thuringiensis, including ecology, cell structure and development, virulence attributes, gene regulation and genetic exchange systems, and experimental models of disease.
Figures



References
-
- Lapidus A, Goltsman E, Auger S, Galleron N, Ségurens B, Dossat C, Land ML, Broussolle V, Brillard J, Guinebretiere MH, Sanchis V, Nguen-The C, Lereclus D, Richardson P, Wincker P, Weissenbach J, Ehrlich SD, Sorokin A. 2008. Extending the Bacillus cereus group genomics to putative food-borne pathogens of different toxicity. Chem Biol Interact 171:236–249 10.1016/j.cbi.2007.03.003. [PubMed] - DOI - PubMed
-
- Kolstø AB, Lereclus D, Mock M. 2002. Genome structure and evolution of the Bacillus cereus group. Curr Top Microbiol Immunol 264:95–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

