Vaccine Approaches To Protect against Group A Streptococcal Pharyngitis
- PMID: 31111819
- PMCID: PMC11026073
- DOI: 10.1128/microbiolspec.GPP3-0010-2018
Vaccine Approaches To Protect against Group A Streptococcal Pharyngitis
Abstract
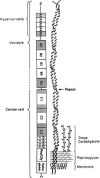
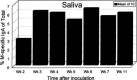
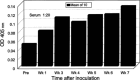
Streptococcal pharyngitis (or strep throat) is a common childhood disease affecting millions of children each year, but it is one of the only childhood diseases for which a vaccine does not exist. While for decades the development of a vaccine has been the center of attention in many laboratories worldwide, with some successes, no corporate development has yet to be initiated. The reason for this probably lies in our inability to conclusively identify the streptococcal molecule or molecules responsible for the heart cross-reactive antibodies observed in the serum of rheumatic fever patients. Without this specific knowledge, any streptococcal vaccine antigen is suspect and thus not the target for a billion-dollar investment, despite the fact that the exact role of cross-reactive antibodies in rheumatic fever is still questionable. This article will describe the development of several approaches to protect against Streptococcus pyogenes infections over the past several decades.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

