Testicular tissue cryopreservation: 8 years of experience from a coordinated network of academic centers
- PMID: 31111889
- PMCID: PMC6554046
- DOI: 10.1093/humrep/dez043
Testicular tissue cryopreservation: 8 years of experience from a coordinated network of academic centers
Abstract
Study question: Is it feasible to disseminate testicular tissue cryopreservation with a standardized protocol through a coordinated network of centers and provide centralized processing/freezing for centers that do not have those capabilities?
Summary answer: Centralized processing and freezing of testicular tissue from multiple sites is feasible and accelerates recruitment, providing the statistical power to make inferences that may inform fertility preservation practice.
What is known already: Several centers in the USA and abroad are preserving testicular biopsies for patients who cannot preserve sperm in anticipation that cell- or tissue-based therapies can be used in the future to generate sperm and offspring.
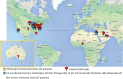
Study design, size, duration: Testicular tissue samples from 189 patients were cryopreserved between January 2011 and November 2018. Medical diagnosis, previous chemotherapy exposure, tissue weight, and presence of germ cells were recorded.
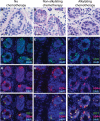
Participants/materials, setting, methods: Human testicular tissue samples were obtained from patients undergoing treatments likely to cause infertility. Twenty five percent of the patient's tissue was donated to research and 75% was stored for patient's future use. The tissue was weighed, and research tissue was fixed for histological analysis with Periodic acid-Schiff hematoxylin staining and/or immunofluorescence staining for DEAD-box helicase 4, and/or undifferentiated embryonic cell transcription factor 1.
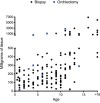
Main results and the role of chance: The average age of fertility preservation patients was 7.9 (SD = 5) years and ranged from 5 months to 34 years. The average amount of tissue collected was 411.3 (SD = 837.3) mg and ranged from 14.4 mg-6880.2 mg. Malignancies (n = 118) were the most common indication for testicular tissue freezing, followed by blood disorders (n = 45) and other conditions (n = 26). Thirty nine percent (n = 74) of patients had initiated their chemotherapy prior to undergoing testicular biopsy. Of the 189 patients recruited to date, 137 have been analyzed for the presence of germ cells and germ cells were confirmed in 132.
Limitations, reasons for caution: This is a descriptive study of testicular tissues obtained from patients who were at risk of infertility. The function of spermatogonia in those biopsies could not be tested by transplantation due limited sample size.
Wider implications of the findings: Patients and/or guardians are willing to pursue an experimental fertility preservation procedure when no alternatives are available. Our coordinated network of centers found that many patients request fertility preservation after initiating gonadotoxic therapies. This study demonstrates that undifferentiated stem and progenitor spermatogonia may be recovered from the testicular tissues of patients who are in the early stages of their treatment and have not yet received an ablative dose of therapy. The function of those spermatogonia was not tested.
Study funding/competing interest(s): Support for the research was from the Eunice Kennedy Shriver National Institute for Child Health and Human Development grants HD061289 and HD092084, the Scaife Foundation, the Richard King Mellon Foundation, the Departments of Ob/Gyn & Reproductive Sciences and Urology of the University of Pittsburgh Medical Center, United States-Israel Binational Science Foundation (BSF), and the Kahn Foundation. The authors declare that they do not have competing financial interests.
Keywords: Spermatogonial stem cells; fertility preservation; spermatogenesis; testicular tissue cryopreservation; testis.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology.
Figures

References
-
- Bahadur G, Chatterjee R, Ralph D. Testicular tissue cryopreservation in boys. Ethical and legal issues: case report. Hum Reprod 2000;15:1416–1420. - PubMed
-
- Bak CW, Seok HH, Song SH, Kim ES, Her YS, Yoon TK. Hormonal imbalances and psychological scars left behind in infertile men. J Androl 2012;33:181–189. - PubMed
-
- Blatt J, Mulvihill JJ, Ziegler JL, Young RC, Poplack DG. Pregnancy outcome following cancer chemotherapy. Am J Med 1980;69:828–832. - PubMed
-
- Brannigan RE, Sandlow JI. Cryopreservation of sperm after chemotherapy. J Androl 2008;29:e1–e2. - PubMed
-
- Brook PF, Radford JA, Shalet SM, Joyce AD, Gosden RG. Isolation of germ cells from human testicular tissue for low temperature storage and autotransplantation. Fertil Steril 2001;75:269–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

