Nanoscale integration of single cell biologics discovery processes using optofluidic manipulation and monitoring
- PMID: 31112285
- PMCID: PMC6771990
- DOI: 10.1002/bit.27024
Nanoscale integration of single cell biologics discovery processes using optofluidic manipulation and monitoring
Abstract
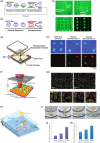
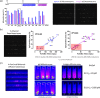
The new and rapid advancement in the complexity of biologics drug discovery has been driven by a deeper understanding of biological systems combined with innovative new therapeutic modalities, paving the way to breakthrough therapies for previously intractable diseases. These exciting times in biomedical innovation require the development of novel technologies to facilitate the sophisticated, multifaceted, high-paced workflows necessary to support modern large molecule drug discovery. A high-level aspiration is a true integration of "lab-on-a-chip" methods that vastly miniaturize cellulmical experiments could transform the speed, cost, and success of multiple workstreams in biologics development. Several microscale bioprocess technologies have been established that incrementally address these needs, yet each is inflexibly designed for a very specific process thus limiting an integrated holistic application. A more fully integrated nanoscale approach that incorporates manipulation, culture, analytics, and traceable digital record keeping of thousands of single cells in a relevant nanoenvironment would be a transformative technology capable of keeping pace with today's rapid and complex drug discovery demands. The recent advent of optical manipulation of cells using light-induced electrokinetics with micro- and nanoscale cell culture is poised to revolutionize both fundamental and applied biological research. In this review, we summarize the current state of the art for optical manipulation techniques and discuss emerging biological applications of this technology. In particular, we focus on promising prospects for drug discovery workflows, including antibody discovery, bioassay development, antibody engineering, and cell line development, which are enabled by the automation and industrialization of an integrated optoelectronic single-cell manipulation and culture platform. Continued development of such platforms will be well positioned to overcome many of the challenges currently associated with fragmented, low-throughput bioprocess workflows in biopharma and life science research.
Keywords: advanced biotechnology; bioassay development; digital cell biology; drug discovery; nanoscale cell culture; optical manipulation techniques; single cell technology.
© 2019 The Authors. Biotechnology and Bioengineering Published by Wiley Periodicals, Inc.
Figures





References
-
- Ashkin, A. (1970). Acceleration and trapping of particles by radiation pressure. Physical Review Letters, 24(4), 156–159. 10.1103/physrevlett.24.156 - DOI
-
- Ashkin, A. , Dziedzic, J. M. , Bjorkholm, J. E. , & Chu, S. (1986). Observation of a single‐beam gradient force optical trap for dielectric particles. Optics Letters, 11(5), 288 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

