Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome
- PMID: 31112383
- PMCID: PMC6741345
- DOI: 10.1056/NEJMoa1901686
Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome
Abstract
Background: The benefits of early continuous neuromuscular blockade in patients with acute respiratory distress syndrome (ARDS) who are receiving mechanical ventilation remain unclear.
Methods: We randomly assigned patients with moderate-to-severe ARDS (defined by a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen of <150 mm Hg with a positive end-expiratory pressure [PEEP] of ≥8 cm of water) to a 48-hour continuous infusion of cisatracurium with concomitant deep sedation (intervention group) or to a usual-care approach without routine neuromuscular blockade and with lighter sedation targets (control group). The same mechanical-ventilation strategies were used in both groups, including a strategy involving a high PEEP. The primary end point was in-hospital death from any cause at 90 days.
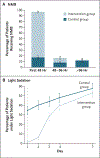
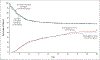
Results: The trial was stopped at the second interim analysis for futility. We enrolled 1006 patients early after the onset of moderate-to-severe ARDS (median, 7.6 hours after onset). During the first 48 hours after randomization, 488 of the 501 patients (97.4%) in the intervention group started a continuous infusion of cisatracurium (median duration of infusion, 47.8 hours; median dose, 1807 mg), and 86 of the 505 patients (17.0%) in the control group received a neuromuscular blocking agent (median dose, 38 mg). At 90 days, 213 patients (42.5%) in the intervention group and 216 (42.8%) in the control group had died before hospital discharge (between-group difference, -0.3 percentage points; 95% confidence interval, -6.4 to 5.9; P = 0.93). While in the hospital, patients in the intervention group were less physically active and had more adverse cardiovascular events than patients in the control group. There were no consistent between-group differences in end points assessed at 3, 6, and 12 months.
Conclusions: Among patients with moderate-to-severe ARDS who were treated with a strategy involving a high PEEP, there was no significant difference in mortality at 90 days between patients who received an early and continuous cisatracurium infusion and those who were treated with a usual-care approach with lighter sedation targets. (Funded by the National Heart, Lung, and Blood Institute; ROSE ClinicalTrials.gov number, NCT02509078.).
Copyright © 2019 Massachusetts Medical Society.
Figures
Comment in
-
Early Paralytic Agents for ARDS? Yes, No, and Sometimes.N Engl J Med. 2019 May 23;380(21):2061-2063. doi: 10.1056/NEJMe1905627. Epub 2019 May 19. N Engl J Med. 2019. PMID: 31112382 No abstract available.
-
Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome.N Engl J Med. 2019 Aug 22;381(8):785. doi: 10.1056/NEJMc1908874. N Engl J Med. 2019. PMID: 31433931 No abstract available.
-
Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome.N Engl J Med. 2019 Aug 22;381(8):785-786. doi: 10.1056/NEJMc1908874. N Engl J Med. 2019. PMID: 31433932 No abstract available.
-
Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome.N Engl J Med. 2019 Aug 22;381(8):786-787. doi: 10.1056/NEJMc1908874. N Engl J Med. 2019. PMID: 31433933 No abstract available.
-
Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome.N Engl J Med. 2019 Aug 22;381(8):787. doi: 10.1056/NEJMc1908874. N Engl J Med. 2019. PMID: 31433934 No abstract available.
-
Neuromuscular blocking agents for acute respiratory distress syndrome: how did we get conflicting results?Crit Care. 2019 Sep 6;23(1):305. doi: 10.1186/s13054-019-2586-3. Crit Care. 2019. PMID: 31492197 Free PMC article. No abstract available.
-
Probability of benefit with the use of neuromuscular blockade in patients with acute respiratory distress syndrome.J Thorac Dis. 2019 Sep;11(9):3676-3680. doi: 10.21037/jtd.2019.09.26. J Thorac Dis. 2019. PMID: 31656637 Free PMC article. No abstract available.
-
Early neuromuscular blockade in moderate to severe acute respiratory distress syndrome: do not throw the baby out with the bathwater!J Thorac Dis. 2019 Nov;11(11):E231-E234. doi: 10.21037/jtd.2019.10.25. J Thorac Dis. 2019. PMID: 31903290 Free PMC article. No abstract available.
-
Early neuromuscular blockade in acute respiratory distress syndrome: to personalize or paralyze?J Thorac Dis. 2019 Dec;11(12):5701-5705. doi: 10.21037/jtd.2019.12.101. J Thorac Dis. 2019. PMID: 32030306 Free PMC article. No abstract available.
-
Revisiting Old Friends: Adjunctive Therapies in Acute Respiratory Distress Syndrome.Am J Respir Crit Care Med. 2021 Aug 15;204(4):473-475. doi: 10.1164/rccm.202009-3722RR. Am J Respir Crit Care Med. 2021. PMID: 34192505 Free PMC article. No abstract available.
References
-
- The ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307: 2526–33. - PubMed
-
- Slutsky AS. Neuromuscular blocking agents in ARDS. N Engl J Med 2010; 363: 1176–80. - PubMed
-
- Price DR, Mikkelsen ME, Umscheid CA, Armstrong EJ. Neuromuscular blocking agents and neuromuscular dysfunction acquired in critical illness: a systematic review and meta-analysis. Crit Care Med 2016; 44: 2070–8. - PubMed
-
- Puthucheary Z, Rawal J, Ratnayake G, Harridge S, Montgomery H, Hart N. Neuromuscular blockade and skeletal muscle weakness in critically ill patients: time to rethink the evidence? Am J Respir Crit Care Med 2012; 185: 911–7. - PubMed
-
- Papazian L, Forel J-M, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363: 1107–16. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 HL123018/HL/NHLBI NIH HHS/United States
- U01 HL123031/HL/NHLBI NIH HHS/United States
- U01HL123031/HL/NHLBI NIH HHS/United States
- U01 HL122989/HL/NHLBI NIH HHS/United States
- U01HL123010/HL/NHLBI NIH HHS/United States
- U01HL123027/HL/NHLBI NIH HHS/United States
- U01HL123018/HL/NHLBI NIH HHS/United States
- U01HL123009/HL/NHLBI NIH HHS/United States
- U01 HL123004/HL/NHLBI NIH HHS/United States
- U01 HL123008/HL/NHLBI NIH HHS/United States
- U01 HL122998/HL/NHLBI NIH HHS/United States
- U01HL123004/HL/NHLBI NIH HHS/United States
- U01HL122989/HL/NHLBI NIH HHS/United States
- U01HL123023/HL/NHLBI NIH HHS/United States
- K23 HL143053/HL/NHLBI NIH HHS/United States
- U01 HL123020/HL/NHLBI NIH HHS/United States
- U01HL123020/HL/NHLBI NIH HHS/United States
- U01 HL123009/HL/NHLBI NIH HHS/United States
- U01HL122998/HL/NHLBI NIH HHS/United States
- U01 HL123022/HL/NHLBI NIH HHS/United States
- U01HL123008/HL/NHLBI NIH HHS/United States
- U01 HL123023/HL/NHLBI NIH HHS/United States
- U01HL123022/HL/NHLBI NIH HHS/United States
- U01HL123033/HL/NHLBI NIH HHS/United States
- U01 HL123027/HL/NHLBI NIH HHS/United States
- U01 HL123033/HL/NHLBI NIH HHS/United States
- U01 HL123010/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
