Mometasone or Tiotropium in Mild Asthma with a Low Sputum Eosinophil Level
- PMID: 31112384
- PMCID: PMC6711475
- DOI: 10.1056/NEJMoa1814917
Mometasone or Tiotropium in Mild Asthma with a Low Sputum Eosinophil Level
Abstract
Background: In many patients with mild, persistent asthma, the percentage of eosinophils in sputum is less than 2% (low eosinophil level). The appropriate treatment for these patients is unknown.
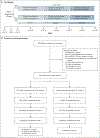
Methods: In this 42-week, double-blind, crossover trial, we assigned 295 patients who were at least 12 years of age and who had mild, persistent asthma to receive mometasone (an inhaled glucocorticoid), tiotropium (a long-acting muscarinic antagonist), or placebo. The patients were categorized according to the sputum eosinophil level (<2% or ≥2%). The primary outcome was the response to mometasone as compared with placebo and to tiotropium as compared with placebo among patients with a low sputum eosinophil level who had a prespecified differential response to one of the trial agents. The response was determined according to a hierarchical composite outcome that incorporated treatment failure, asthma control days, and the forced expiratory volume in 1 second; a two-sided P value of less than 0.025 denoted statistical significance. A secondary outcome was a comparison of results in patients with a high sputum eosinophil level and those with a low level.
Results: A total of 73% of the patients had a low eosinophil level; of these patients, 59% had a differential response to a trial agent. However, there was no significant difference in the response to mometasone or tiotropium, as compared with placebo. Among the patients with a low eosinophil level who had a differential treatment response, 57% (95% confidence interval [CI], 48 to 66) had a better response to mometasone, and 43% (95% CI, 34 to 52) had a better response to placebo (P = 0.14). In contrast 60% (95% CI, 51 to 68) had a better response to tiotropium, whereas 40% (95% CI, 32 to 49) had a better response to placebo (P = 0.029). Among patients with a high eosinophil level, the response to mometasone was significantly better than the response to placebo (74% vs. 26%) but the response to tiotropium was not (57% vs. 43%).
Conclusions: The majority of patients with mild, persistent asthma had a low sputum eosinophil level and had no significant difference in their response to either mometasone or tiotropium as compared with placebo. These data provide equipoise for a clinically directive trial to compare an inhaled glucocorticoid with other treatments in patients with a low eosinophil level. (Funded by the National Heart, Lung, and Blood Institute; SIENA ClinicalTrials.gov number, NCT02066298.).
Copyright © 2019 Massachusetts Medical Society.
Figures

Comment in
-
How Should We Treat Patients with Mild Asthma?N Engl J Med. 2019 May 23;380(21):2064-2066. doi: 10.1056/NEJMe1905354. Epub 2019 May 19. N Engl J Med. 2019. PMID: 31112381 No abstract available.
-
[Treatment of mild asthma : Steroids in Eosinophil Negative Asthma (SIENA) and Novel Symbicort Turbuhaler Asthma Reliever Therapy (Novel START)].Internist (Berl). 2019 Dec;60(12):1328-1332. doi: 10.1007/s00108-019-00688-w. Internist (Berl). 2019. PMID: 31664463 German. No abstract available.
References
-
- Martin RJ, Szefler SJ, Chinchilli VM, et al. Systemic effect comparisons of six inhaled corticosteroid preparations. Am J Respir Crit Care Med 2002;165:1377–83. - PubMed
-
- Deykin A, Lazarus SC, Fahy JV, et al. Sputum eosinophil counts predict asthma control after discontinuation of inhaled corticosteroids. J Allergy Clin Immunol 2005;115:720–7. - PubMed
-
- Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology 2006;11:54–61. - PubMed
-
- Haldar P, Pavord ID. Noneosinophilic asthma: a distinct clinical and pathologic phenotype. J Allergy Clin Immunol 2007; 119:1043–52. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HL098102/HL/NHLBI NIH HHS/United States
- K23 AI125785/AI/NIAID NIH HHS/United States
- U10 HL098115/HL/NHLBI NIH HHS/United States
- HL098096/HL/NHLBI NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- HL098115/HL/NHLBI NIH HHS/United States
- U10 HL098103/HL/NHLBI NIH HHS/United States
- HL098103/HL/NHLBI NIH HHS/United States
- HL098107/HL/NHLBI NIH HHS/United States
- HL098177/HL/NHLBI NIH HHS/United States
- U10 HL064313/HL/NHLBI NIH HHS/United States
- HL098098/HL/NHLBI NIH HHS/United States
- U10 HL098107/HL/NHLBI NIH HHS/United States
- HL0981 12/HL/NHLBI NIH HHS/United States
- HL098090/HL/NHLBI NIH HHS/United States
- HL098075/HL/NHLBI NIH HHS/United States
- U10 HL098075/HL/NHLBI NIH HHS/United States
