Positron Emission Tomography Score Has Greater Prognostic Significance Than Pretreatment Risk Stratification in Early-Stage Hodgkin Lymphoma in the UK RAPID Study
- PMID: 31112475
- PMCID: PMC6638600
- DOI: 10.1200/JCO.18.01799
Positron Emission Tomography Score Has Greater Prognostic Significance Than Pretreatment Risk Stratification in Early-Stage Hodgkin Lymphoma in the UK RAPID Study
Abstract
Purpose: Accurate stratification of patients is an important goal in Hodgkin lymphoma (HL), but the role of pretreatment clinical risk stratification in the context of positron emission tomography (PET) -adapted treatment is unclear. We performed a subsidiary analysis of the RAPID trial to assess the prognostic value of pretreatment risk factors and PET score in determining outcomes.
Patients and methods: Patients with stage IA to IIA HL and no mediastinal bulk underwent PET assessment after three cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine; 143 PET-positive patients (PET score, 3 to 5) received a fourth doxorubicin, bleomycin, vinblastine, and dacarbazine cycle and involved-field radiotherapy, and 419 patients in complete metabolic remission were randomly assigned to receive involved-field radiotherapy (n = 208) or no additional treatment (n = 211). Cox regression was used to investigate the association between PET score and pretreatment risk factors with HL-specific event-free survival (EFS).
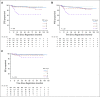
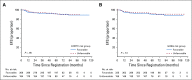
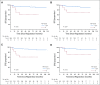
Results: High PET score was associated with inferior EFS, before (P < .001) and after adjustment (P = .01) for baseline risk stratification. Only patients with a postchemotherapy PET score of 5 (uptake ≥ three times maximum liver uptake) had an increased risk of progression or HL-related death (hazard ratio, 9.4 v score of 3; 95% CI, 2.8 to 31.3 and hazard ratio, 6.7 v score of 4; 95% CI, 1.4 to 31.7). Patients with a PET score of 5 also had inferior progression-free and overall survival. There was no association between European Organisation for Research and Treatment of Cancer or German Hodgkin Study Group risk group and EFS, before or after adjusting for PET score (all P > .4).
Conclusion: In RAPID, a positive PET scan did not carry uniform prognostic weight; only a PET score of 5 was associated with inferior outcomes. This suggests that in future trials involving patients without B symptoms or mediastinal bulk, a score of 5 rather than a positive PET result should be used to guide treatment escalation in early-stage HL.
Trial registration: ClinicalTrials.gov NCT00943423.
Figures

Comment in
-
Only a Small Proportion of Patients With Early-Stage Hodgkin Lymphoma May Potentially Benefit From Fluorodeoxyglucose-Positron Emission Tomography-Adapted Treatment Escalation.J Clin Oncol. 2019 Dec 1;37(34):3323. doi: 10.1200/JCO.19.01491. Epub 2019 Sep 30. J Clin Oncol. 2019. PMID: 31566991 No abstract available.
-
Reply to H.J.A. Adams et al and C. Kobe et al.J Clin Oncol. 2019 Dec 1;37(34):3325-3326. doi: 10.1200/JCO.19.02056. Epub 2019 Sep 30. J Clin Oncol. 2019. PMID: 31566994 No abstract available.
-
Predictive Value of Positron Emission Tomography/Computed Tomography After ABVD-Based Chemotherapy in Early-Stage Hodgkin Lymphoma.J Clin Oncol. 2019 Dec 1;37(34):3324-3325. doi: 10.1200/JCO.19.01761. Epub 2019 Sep 30. J Clin Oncol. 2019. PMID: 31566995 No abstract available.
References
-
- Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med. 2015;373:2499–2511. - PubMed
-
- Raemaekers JMM, Andre MPE, Federico M, et al: Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 32:1188-1194, 2014. - PubMed
-
- Behringer K, Goergen H, Hitz F, et al. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin’s lymphoma (GHSG HD13): An open-label, randomised, non-inferiority trial. Lancet. 2015;385:1418–1427. - PubMed
-
- von Tresckow B, Plütschow A, Fuchs M, et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: Final analysis of the German Hodgkin Study Group HD14 trial. J Clin Oncol. 2012;30:907–913. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

