Three-dimensional label-free imaging throughout adipocyte differentiation by stimulated Raman microscopy
- PMID: 31112567
- PMCID: PMC6528968
- DOI: 10.1371/journal.pone.0216811
Three-dimensional label-free imaging throughout adipocyte differentiation by stimulated Raman microscopy
Abstract
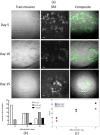
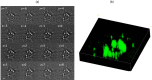
Lipid droplets are lipid-storage organelles with a key role in lipid accumulation pathologies such as diabetes, obesity and atherosclerosis. Despite their important functions many aspects of lipid droplets biology are still unknown. This is partially due to the current use of exogenous labels to monitor their formation and remodelling by invasive imaging methods. Here, we apply stimulated Raman scattering microscopy to acquire images with high spatial resolution along with resolving capabilities of lipids and proteins and three-dimensional sectioning. Our images and data analysis demonstrate an increase in the number of large (>15μm2) lipid droplets in human adipocyte cells during differentiation process. In addition, spatially-resolved maps of lipids and proteins inside cells and three dimensional reconstructions of lipids at the initial and final steps of adipocyte differentiation are reported, too.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

