Inhibition of immune checkpoints PD-1, CTLA-4, and IDO1 coordinately induces immune-mediated liver injury in mice
- PMID: 31112568
- PMCID: PMC6528985
- DOI: 10.1371/journal.pone.0217276
Inhibition of immune checkpoints PD-1, CTLA-4, and IDO1 coordinately induces immune-mediated liver injury in mice
Abstract
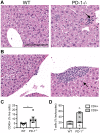
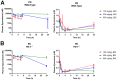
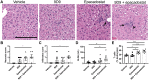
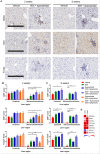
Cancer cells harness immune checkpoints such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1) and indoleamine 2,3-dioxygenase 1 (IDO1) to evade immune control. Checkpoint inhibitors have demonstrated durable anti-tumor efficacy in human and preclinical models. Liver toxicity is one of the common immune-related adverse events associated with checkpoint inhibitors (CPIs) and its frequency and severity often increase significantly during CPI combination therapies. We aim to develop a mouse model to elucidate the immune mechanisms of CPI-associated liver toxicity. Co-administration of CTLA-4 blocking antibody, 9D9, and/or an IDO1 inhibitor, epacadostat in wild-type and PD-1-/- mice (to simulate the effect of PD1 blockade) synergistically induced liver injury and immune cell infiltration. Infiltrated cells were primarily composed of CD8+ T cells and positively associated with hepatocyte necrosis. Strikingly, sites of hepatocyte necrosis were frequently surrounded by clusters of mononuclear immune cells. CPI treatments resulted in increased expression of genes associated with hepatocyte cell death, leukocyte migration and T cell activation in the liver. In conclusion, blockade of immune checkpoints PD-1, CTLA-4, and IDO1 act synergistically to enhance T cell infiltration and activity in the liver, leading to hepatocyte death.
Conflict of interest statement
Authors TA, HL, DB, QZ, SX, VT and CJ are employed by Pfizer Inc. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–502. Epub 2017/04/25. 10.1016/S0140-6736(17)31046-2 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

