Epigenetic gene regulation, chromatin structure, and force-induced chromatin remodelling in epidermal development and homeostasis
- PMID: 31112907
- PMCID: PMC8259782
- DOI: 10.1016/j.gde.2019.04.014
Epigenetic gene regulation, chromatin structure, and force-induced chromatin remodelling in epidermal development and homeostasis
Abstract
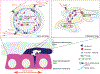
The skin epidermis is a constantly renewing stratified epithelium that provides essential protective barrier functions throughout life. Epidermal stratification is governed by a step-wise differentiation program that requires precise spatiotemporal control of gene expression. How epidermal self-renewal and differentiation are regulated remains a fundamental open question. Cell-intrinsic and cell-extrinsic mechanisms that modify chromatin structure and interactions have been identified as key regulators of epidermal differentiation and stratification. Here, we will review the recent advances in our understanding of how chromatin modifiers, tissue-specific transcription factors, and force-induced nuclear remodeling processes function to shape chromatin and to control epidermal tissue development and homeostasis.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures
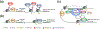
References
-
- Bickmore WA and Van Steensel B (2013) Genome architecture: Domain organization of interphase chromosomes. Cell 152, 1270–1284. - PubMed
-
- Le HQ et al. (2016) Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol 18, 864–75 - PubMed
-
** This paper demonstrates how extrinsic mechanical strain impacts chromatin structure and epigenetic gene regulation. The authors show that while strain induces general chromatin decompaction in epidermal progenitor cells, transcriptional repression leads to accumulation of H3K27me3 at terminal differentiation genes, preventing their expression.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

