Oncologic Outcomes in Patients Who Undergo Neoadjuvant Chemoradiotherapy and Total Mesorectal Excision for Locally Advanced Rectal Cancer: A 14-Year Experience in a Single Institution
- PMID: 31113173
- PMCID: PMC6529756
- DOI: 10.3393/ac.2019.04.22.1
Oncologic Outcomes in Patients Who Undergo Neoadjuvant Chemoradiotherapy and Total Mesorectal Excision for Locally Advanced Rectal Cancer: A 14-Year Experience in a Single Institution
Abstract
Purpose: This study evaluated the oncologic outcomes of locally advanced rectal cancer patients who underwent preoperative neoadjuvant chemoradiotherapy (CRT) followed by surgery and determined the prognostic significance of pathologic complete response (pCR).
Methods: Between January 2002 and December 2015, 580 patients with rectal cancer who underwent surgery after neoadjuvant CRT were identified. Survival according to tumor response to CRT and pathologic stage was analyzed using the Kaplan-Meier method, and the Cox proportional hazard model was used to identify factors associated with survival outcomes.
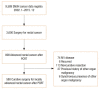
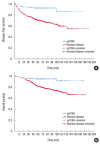
Results: A total of 111 patients (23.7%) achieved pCR while the other 469 patients showed residual disease. Patients with pCR had a lower pretreatment carcinoembryonic antigen level and earlier cT classification than those with residual disease. With a median follow-up of 78 months, disease-free survival (DFS) and overall survival (OS) were significantly better in the pCR group than in the residual disease group. The 5-year DFS and 5-year OS for patients with ypStage 0, I, II, or III were 92.5%, 85.1%, 72.2%, 54.3% (P < 0.001) and 94.5%, 91.0%, 83.1%, 69.3%, respectively (P < 0.001). Pathologic AJCC stage after CRT was the most statistically significant independent predictor of OS (HR, 6.97 [95% confidence interval, 3.16-15.39] for stage III vs. stage 0) and DFS (HR, 7.30 [95% confidence interval, 3.63-14.67] for stage III vs. stage 0).
Conclusion: Rectal cancer patients who achieved pCR showed improved survival compared to those with residual disease after preoperative CRT. Moreover, pCR was an independent indicator of OS and DFS, and pathologic AJCC stage was correlated with survival after preoperative CRT.
Keywords: Chemoradiotherapy; Pathologic complete response; Rectal neoplasms; Survival.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- National Comprehensive Cancer Network . Fort Wathington (PA): National Comprehensive Cancer Network; 2018. NCCN clinical practice guidelines in oncology (NCCN Guidelines): rectal cancer (Version 3.2018) [Internet] [cited 2019 Feb 6]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.
-
- van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–82. - PubMed
-
- Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

