Panitumumab Induced Forearm Panniculitis in Two Women With Metastatic Colon Cancer
- PMID: 31113346
- PMCID: PMC6864607
- DOI: 10.2174/1574886314666190522094713
Panitumumab Induced Forearm Panniculitis in Two Women With Metastatic Colon Cancer
Abstract
Background: Panitumumab is an EGFR inhibitor used for the treatment of metastatic colorectal cancer (mCRC), even if its use is related to skin toxicity.
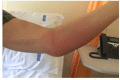
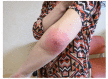
Case presentation: We report the development of forearm panniculitis in two women during the treatment with Panitumumab (6 mg/Kg intravenous every 2 weeks) + FOLFOX-6 (leucovorin, 5- fluorouracil, and oxaliplatin at higher dosage) for the treatment of mCRC.
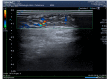
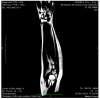
Results: In both patients, clinical, laboratory and radiological evaluation documented the presence of a local panniculitis, probably related to panitumumab (Naranjo score: 6). Panatimumab discontinuation and antimicrobial + corticosteroid treatment induced a remission of skin manifestations.
Conclusion: We reported for the first time the development of panniculitis during Panitumumab treatment, and we documented that the treatment with beta-lactams to either fluoroquinolones or oxazolidinone in the presence of corticosteroid improves clinical symptoms in young patients with mCRC, without the development of adverse drug reactions or drug-drug interactions.
Keywords: EGFR; FOLFOX; Panitumumab; adverse drug reaction; mCRC; panniculitis..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures
References
-
- Ra H.S., Shin S.J., Kim J.H., Lim H., Cho B.C., Roh M.R. The impact of dermatological toxicities of anti-cancer therapy on the dermatological quality of life of cancer patients. J. Eur. Acad. Dermatol. Venereol. 2013;27(1):e53–e59. - PubMed
-
- Rosen A.C., Case E.C., Dusza S.W., et al. Impact of dermatologic adverse events on quality of life in 283 cancer patients: A questionnaire study in a dermatology referral clinic. Am. J. Clin. Dermatol. 2013;14(4):327–333. - PubMed
-
- Douillard J.Y., Oliner K.S., Siena S., et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 2013;369(11):1023–1034. - PubMed
-
- Schwartzberg L.S., Rivera F., Karthaus M., et al. PEAK: A randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J. Clin. Oncol. 2014;32(21):2240–2247. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

