Immunogenetic response of the bananaquit in the face of malarial parasites
- PMID: 31113360
- PMCID: PMC6529992
- DOI: 10.1186/s12862-019-1435-y
Immunogenetic response of the bananaquit in the face of malarial parasites
Abstract
Background: In the arms race between hosts and parasites, genes involved in the immune response are targets for natural selection. Toll-Like Receptor (TLR) genes play a role in parasite detection as part of the innate immune system whereas Major Histocompatibility Complex (MHC) genes encode proteins that display antigens as part of the vertebrate adaptive immune system. Thus, both gene families are under selection pressure from pathogens. The bananaquit (Coereba flaveola) is a passerine bird that is a common host of avian malarial parasites (Plasmodium sp. and Haemoproteus sp.). We assessed molecular variation of TLR and MHC genes in a wild population of bananaquits and identified allelic associations with resistance/susceptibility to parasitic infection to address hypotheses of avian immune response to haemosporidian parasites.
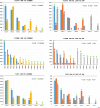
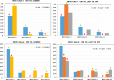
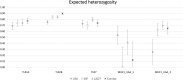
Results: We found that allele frequencies are associated with infection status at the immune loci studied. A consistent general trend showed the infected groups possessed more alleles at lower frequencies, and exhibited unique alleles, compared to the uninfected group.
Conclusions: Our results support the theory of natural selection favoring particular alleles for resistance while maintaining overall genetic diversity in the population, a mechanism which has been demonstrated in some systems in MHC previously but understudied in TLRs.
Keywords: Avian malaria; Haemosporidian parasites; Major histocompatibility complex; Toll-like receptor.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Higher body condition with infection by Haemoproteus parasites in Bananaquits (Coereba flaveola).PeerJ. 2024 Mar 29;12:e16361. doi: 10.7717/peerj.16361. eCollection 2024. PeerJ. 2024. PMID: 38563018 Free PMC article.
-
The genome sequence and insights into the immunogenetics of the bananaquit (Passeriformes: Coereba flaveola).Immunogenetics. 2017 Mar;69(3):175-186. doi: 10.1007/s00251-016-0960-8. Epub 2016 Nov 25. Immunogenetics. 2017. PMID: 27888301
-
Blood parasites shape extreme major histocompatibility complex diversity in a migratory passerine.Mol Ecol. 2018 Jun;27(11):2594-2603. doi: 10.1111/mec.14592. Epub 2018 May 15. Mol Ecol. 2018. PMID: 29654666
-
Evolutionary ecology, taxonomy, and systematics of avian malaria and related parasites.Acta Trop. 2020 Apr;204:105364. doi: 10.1016/j.actatropica.2020.105364. Epub 2020 Jan 30. Acta Trop. 2020. PMID: 32007445 Review.
-
Do avian malaria parasites reduce vector longevity?Curr Opin Insect Sci. 2018 Aug;28:113-117. doi: 10.1016/j.cois.2018.08.001. Epub 2018 Aug 25. Curr Opin Insect Sci. 2018. PMID: 30551761 Review.
Cited by
-
Leveraging an existing whole-genome resequencing population data set to characterize toll-like receptor gene diversity in a threatened bird.Mol Ecol Resour. 2022 Oct;22(7):2810-2825. doi: 10.1111/1755-0998.13656. Epub 2022 Jun 14. Mol Ecol Resour. 2022. PMID: 35635119 Free PMC article.
-
Genetic drift and bottleneck do not influence diversity in Toll-like receptor genes at a small spatial scale in a Himalayan passerine.Ecol Evol. 2020 Oct 15;10(21):12246-12263. doi: 10.1002/ece3.6855. eCollection 2020 Nov. Ecol Evol. 2020. PMID: 33209285 Free PMC article.
-
Extremely low malaria prevalence in a wetland specialist passerine.Parasitology. 2020 Jan;147(1):87-95. doi: 10.1017/S0031182019001215. Epub 2019 Sep 12. Parasitology. 2020. PMID: 31455438 Free PMC article.
-
Higher body condition with infection by Haemoproteus parasites in Bananaquits (Coereba flaveola).PeerJ. 2024 Mar 29;12:e16361. doi: 10.7717/peerj.16361. eCollection 2024. PeerJ. 2024. PMID: 38563018 Free PMC article.
-
Selection Balancing at Innate Immune Genes: Adaptive Polymorphism Maintenance in Toll-Like Receptors.Mol Biol Evol. 2022 May 3;39(5):msac102. doi: 10.1093/molbev/msac102. Mol Biol Evol. 2022. PMID: 35574644 Free PMC article. Review.
References
-
- Gokhale Chaitanya S, Papkou Andrei, Traulsen Arne, Schulenburg Hinrich. Lotka–Volterra dynamics kills the Red Queen: population size fluctuations and associated stochasticity dramatically change host-parasite coevolution. BMC Evolutionary Biology. 2013;13(1):254. doi: 10.1186/1471-2148-13-254. - DOI - PMC - PubMed
-
- Klein J. Natural history of the major histocompatibility complex. New York: Wiley; 1986. p. 775.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

