IGF-1 overexpression improves mesenchymal stem cell survival and promotes neurological recovery after spinal cord injury
- PMID: 31113444
- PMCID: PMC6530133
- DOI: 10.1186/s13287-019-1223-z
IGF-1 overexpression improves mesenchymal stem cell survival and promotes neurological recovery after spinal cord injury
Abstract
Background: Survival and therapeutic actions of bone marrow-derived mesenchymal stem cells (BMMSCs) can be limited by the hostile microenvironment present during acute spinal cord injury (SCI). Here, we investigated whether BMMSCs overexpressing insulin-like growth factor 1 (IGF-1), a cytokine involved in neural development and injury repair, improved the therapeutic effects of BMMSCs in SCI.
Methods: Using a SCI contusion model in C57Bl/6 mice, we transplanted IGF-1 overexpressing or wild-type BMMSCs into the lesion site following SCI and evaluated cell survival, proliferation, immunomodulation, oxidative stress, myelination, and functional outcomes.
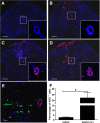
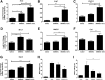
Results: BMMSC-IGF1 transplantation was associated with increased cell survival and recruitment of endogenous neural progenitor cells compared to BMMSC- or saline-treated controls. Modulation of gene expression of pro- and anti-inflammatory mediators was observed after BMMSC-IGF1 and compared to saline- and BMMSC-treated mice. Treatment with BMMSC-IGF1 restored spinal cord redox homeostasis by upregulating antioxidant defense genes. BMMSC-IGF1 protected against SCI-induced myelin loss, showing more compact myelin 28 days after SCI. Functional analyses demonstrated significant gains in BMS score and gait analysis in BMMSC-IGF1, compared to BMMSC or saline treatment.
Conclusions: Overexpression of IGF-1 in BMMSC resulted in increased cell survival, immunomodulation, myelination, and functional improvements, suggesting that IGF-1 facilitates the regenerative actions of BMMSC in acute SCI.
Keywords: Bone marrow-derived mesenchymal stem cells; Gene and cell therapy; IGF-1; Spinal cord injury.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Moya A, Paquet J, Deschepper M, Larochette N, Oudina K, Denoeud C, Bensidhoum M, et al. Mesenchymal stem cell failure to adapt to glucose shortage and rapidly use intracellular energy reserves through glycolysis explains poor cell survival after implantation. Stem Cells. 2018;36(3):363–376. doi: 10.1002/stem.2763. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

