Epigenetic upregulation of FKBP5 by aging and stress contributes to NF-κB-driven inflammation and cardiovascular risk
- PMID: 31113877
- PMCID: PMC6561294
- DOI: 10.1073/pnas.1816847116
Epigenetic upregulation of FKBP5 by aging and stress contributes to NF-κB-driven inflammation and cardiovascular risk
Abstract
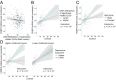
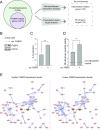
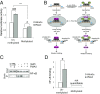
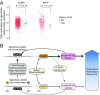
Aging and psychosocial stress are associated with increased inflammation and disease risk, but the underlying molecular mechanisms are unclear. Because both aging and stress are also associated with lasting epigenetic changes, a plausible hypothesis is that stress along the lifespan could confer disease risk through epigenetic effects on molecules involved in inflammatory processes. Here, by combining large-scale analyses in human cohorts with experiments in cells, we report that FKBP5, a protein implicated in stress physiology, contributes to these relations. Across independent human cohorts (total n > 3,000), aging synergized with stress-related phenotypes, measured with childhood trauma and major depression questionnaires, to epigenetically up-regulate FKBP5 expression. These age/stress-related epigenetic effects were recapitulated in a cellular model of replicative senescence, whereby we exposed replicating human fibroblasts to stress (glucocorticoid) hormones. Unbiased genome-wide analyses in human blood linked higher FKBP5 mRNA with a proinflammatory profile and altered NF-κB-related gene networks. Accordingly, experiments in immune cells showed that higher FKBP5 promotes inflammation by strengthening the interactions of NF-κB regulatory kinases, whereas opposing FKBP5 either by genetic deletion (CRISPR/Cas9-mediated) or selective pharmacological inhibition prevented the effects on NF-κB. Further, the age/stress-related epigenetic signature enhanced FKBP5 response to NF-κB through a positive feedback loop and was present in individuals with a history of acute myocardial infarction, a disease state linked to peripheral inflammation. These findings suggest that aging/stress-driven FKBP5-NF-κB signaling mediates inflammation, potentially contributing to cardiovascular risk, and may thus point to novel biomarker and treatment possibilities.
Keywords: FKBP5; aging; epigenetics; inflammation; psychosocial stress.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: E.B.B. receives a research grant from Böhringer-Ingelheim to develop cellular and animal models of enhanced FKBP5 function. She is also coinventor on the following patent application: “FKBP5: a novel target for antidepressant therapy” (European Patent no. EP 1687443 B1).
Figures


References
-
- Niccoli T., Partridge L., Ageing as a risk factor for disease. Curr. Biol. 22, R741–R752 (2012). - PubMed
-
- Blackmore H. L., Ozanne S. E., Programming of cardiovascular disease across the life-course. J. Mol. Cell Cardiol. 83, 122–130 (2015). - PubMed
-
- Danese A., McEwen B. S., Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav. 106, 29–39 (2012). - PubMed
-
- Felitti V. J., et al. , Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) study. Am. J. Prev. Med. 14, 245–258 (1998). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

