Timing the Evolutionary Advent of Cyanobacteria and the Later Great Oxidation Event Using Gene Phylogenies of a Sunscreen
- PMID: 31113897
- PMCID: PMC6529634
- DOI: 10.1128/mBio.00561-19
Timing the Evolutionary Advent of Cyanobacteria and the Later Great Oxidation Event Using Gene Phylogenies of a Sunscreen
Abstract
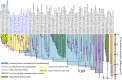
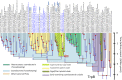
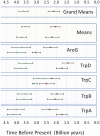
The biosynthesis of the unique cyanobacterial (oxyphotobacterial) indole-phenolic UVA sunscreen, scytonemin, is coded for in a conserved operon that contains both core metabolic genes and accessory, aromatic amino acid biosynthesis genes dedicated to supplying scytonemin's precursors. Comparative genomics shows conservation of this operon in many, but not all, cyanobacterial lineages. Phylogenetic analyses of the operon's aromatic amino acid genes indicate that five of them were recruited into the operon after duplication events of their respective housekeeping cyanobacterial cognates. We combined the fossil record of cyanobacteria and relaxed molecular clock models to obtain multiple estimates of these duplication events, setting a minimum age for the evolutionary advent of scytonemin at 2.1 ± 0.3 billion years. The same analyses were used to estimate the advent of cyanobacteria as a group (and thus the appearance of oxygenic photosynthesis), at 3.6 ± 0.2 billion years before present. Post hoc interpretation of 16S rRNA-based Bayesian analyses was consistent with these estimates. Because of physiological constraints on the use of UVA sunscreens in general, and the biochemical constraints of scytonemin in particular, scytonemin's age must postdate the time when Earth's atmosphere turned oxic, known as the Great Oxidation Event (GOE). Indeed, our biological estimate is in agreement with independent geochemical estimates for the GOE. The difference between the estimated ages of oxygenic photosynthesis and the GOE indicates the long span (on the order of a billion years) of the era of "oxygen oases," when oxygen was available locally but not globally.IMPORTANCE The advent of cyanobacteria, with their invention of oxygenic photosynthesis, and the Great Oxidation Event are arguably among the most important events in the evolutionary history of life on Earth. Oxygen is a significant toxicant to all life, but its accumulation in the atmosphere also enabled the successful development and proliferation of many aerobic organisms, especially metazoans. The currently favored dating of the Great Oxidation Event is based on the geochemical rock record. Similarly, the advent of cyanobacteria is also often drawn from the same estimates because in older rocks paleontological evidence is scarce or has been discredited. Efforts to obtain molecular evolutionary alternatives have offered widely divergent estimates. Our analyses provide a novel means to circumvent these limitations and allow us to estimate the large time gap between the two events.
Keywords: UV photobiology; cyanobacteria; deep evolution; secondary metabolism.
Copyright © 2019 Garcia-Pichel et al.
Figures



Comment in
-
Early Cyanobacteria and the Innovation of Microbial Sunscreens.mBio. 2019 Jun 11;10(3):e01262-19. doi: 10.1128/mBio.01262-19. mBio. 2019. PMID: 31186332 Free PMC article.
Similar articles
-
Geological evidence of oxygenic photosynthesis and the biotic response to the 2400-2200 ma "great oxidation event".Biochemistry (Mosc). 2014 Mar;79(3):165-77. doi: 10.1134/S0006297914030018. Biochemistry (Mosc). 2014. PMID: 24821442 Review.
-
Probing the in vivo biosynthesis of scytonemin, a cyanobacterial ultraviolet radiation sunscreen, through small scale stable isotope incubation studies and MALDI-TOF mass spectrometry.Bioorg Med Chem. 2011 Nov 15;19(22):6620-7. doi: 10.1016/j.bmc.2011.06.005. Epub 2011 Jun 14. Bioorg Med Chem. 2011. PMID: 21742508 Free PMC article.
-
A comparative genomics approach to understanding the biosynthesis of the sunscreen scytonemin in cyanobacteria.BMC Genomics. 2009 Jul 24;10:336. doi: 10.1186/1471-2164-10-336. BMC Genomics. 2009. PMID: 19630972 Free PMC article.
-
The Archean origin of oxygenic photosynthesis and extant cyanobacterial lineages.Proc Biol Sci. 2021 Sep 29;288(1959):20210675. doi: 10.1098/rspb.2021.0675. Epub 2021 Sep 29. Proc Biol Sci. 2021. PMID: 34583585 Free PMC article.
-
Biotechnological Production of the Sunscreen Pigment Scytonemin in Cyanobacteria: Progress and Strategy.Mar Drugs. 2021 Feb 27;19(3):129. doi: 10.3390/md19030129. Mar Drugs. 2021. PMID: 33673485 Free PMC article. Review.
Cited by
-
Expression of Scytonemin Biosynthesis Genes under Alternative Stress Conditions in the Cyanobacterium Nostoc punctiforme.Microorganisms. 2022 Feb 12;10(2):427. doi: 10.3390/microorganisms10020427. Microorganisms. 2022. PMID: 35208882 Free PMC article.
-
The darkest microbiome-a post-human biosphere.Microb Biotechnol. 2022 Jan;15(1):176-185. doi: 10.1111/1751-7915.13976. Epub 2021 Nov 29. Microb Biotechnol. 2022. PMID: 34843168 Free PMC article.
-
Structure revision of scytonemin imine and its relationship to scytonemin chromism and cyanobacterial adaptability.Sci Rep. 2025 Jul 15;15(1):25630. doi: 10.1038/s41598-025-10419-x. Sci Rep. 2025. PMID: 40664929 Free PMC article.
-
Analysis of molecular diversity within single cyanobacterial colonies from environmental samples.Sci Rep. 2020 Oct 28;10(1):18453. doi: 10.1038/s41598-020-75303-2. Sci Rep. 2020. PMID: 33116154 Free PMC article.
-
A novel quinone biosynthetic pathway illuminates the evolution of aerobic metabolism.Proc Natl Acad Sci U S A. 2025 Feb 25;122(8):e2421994122. doi: 10.1073/pnas.2421994122. Epub 2025 Feb 20. Proc Natl Acad Sci U S A. 2025. PMID: 39977315 Free PMC article.
References
-
- Catling DC, Claire MW. 2005. How Earth’s atmosphere evolved to an oxic state: a status report. Earth Planet Sci Lett 237:1–20. doi:10.1016/j.epsl.2005.06.013. - DOI
-
- Knoll AH. 2008. Cyanobacteria and Earth history, p 1–19. In Herrero A, Flores E (ed), The cyanobacteria: molecular biology, genomics, and evolution. Caister Academic Press, Poole, United Kingdom.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

