TFAP2B overexpression contributes to tumor growth and progression of thyroid cancer through the COX-2 signaling pathway
- PMID: 31113934
- PMCID: PMC6529436
- DOI: 10.1038/s41419-019-1600-7
TFAP2B overexpression contributes to tumor growth and progression of thyroid cancer through the COX-2 signaling pathway
Abstract
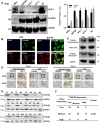
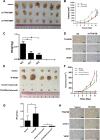
Thyroid cancer is commonly seen in the clinic with a rapidly increasing incidence globally. COX-2 overexpression correlates with the pathologic type of thyroid carcinoma, and it has been suggested that COX-2 overexpression is associated with a poor prognosis. However, little is known about its upstream regulatory mechanism. Bioinformatics suggested that transcription factor AP-2 beta (TFAP2B) might specifically bind to the COX-2 promoter, which was confirmed by biotin-labeled COX-2 promoter pulldown and luciferase reporter assays. We performed western blot and immunohistochemical staining to detect the expression of TFAP2B/COX-2 in thyroid cancer tissues (T) and the matched adjacent noncarcinoma tissues (ANT), and investigated the relationship between TFAP2B/COX-2 expression and clinical pathological factors in thyroid cancer patients. Afterward, MTS, colony formation, cell-apoptosis assay, transwell-invasion and scratch assays were performed to examine the proliferation, apoptosis, invasion, and migration of thyroid cancer cells with TFAP2B knocked down or overexpressed. The mouse xenograft experiment was performed to study in vivo the proliferation of thyroid cancer cells with TFAP2B knocked down or overexpressed. We found that TFAP2B bound to the promoter of COX-2 to activate its expression. Western blot and immunohistochemistry showed that TFAP2B/COX-2 was highly expressed in thyroid cancer, and high TFAP2B and COX-2 expression was associated with aggressive clinicopathological features in thyroid cancer. TFAP2B mediated thyroid cancer cell proliferation, apoptosis, invasion, and migration via the COX-2 signaling pathway in vitro and in vivo. TFAP2B bound to the promoter of COX-2 to activate its expression, indicating that TFAP2B is a critical regulatory molecule in the COX-2 signaling pathway that promoted tumor progression in thyroid cancer.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

