TGFβ-induced cytoskeletal remodeling mediates elevation of cell stiffness and invasiveness in NSCLC
- PMID: 31113982
- PMCID: PMC6529472
- DOI: 10.1038/s41598-019-43409-x
TGFβ-induced cytoskeletal remodeling mediates elevation of cell stiffness and invasiveness in NSCLC
Abstract
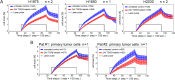
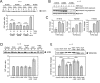
Importance of growth factor (GF) signaling in cancer progression is widely acknowledged. Transforming growth factor beta (TGFβ) is known to play a key role in epithelial-to-mesenchymal transition (EMT) and metastatic cell transformation that are characterized by alterations in cell mechanical architecture and behavior towards a more robust and motile single cell phenotype. However, mechanisms mediating cancer type specific enhancement of cell mechanical phenotype in response to TGFβ remain poorly understood. Here, we combine high-throughput mechanical cell phenotyping, microarray analysis and gene-silencing to dissect cytoskeletal mediators of TGFβ-induced changes in mechanical properties of on-small-cell lung carcinoma (NSCLC) cells. Our experimental results show that elevation of rigidity and invasiveness of TGFβ-stimulated NSCLC cells correlates with upregulation of several cytoskeletal and motor proteins including vimentin, a canonical marker of EMT, and less-known unconventional myosins. Selective probing of gene-silenced cells lead to identification of unconventional myosin MYH15 as a novel mediator of elevated cell rigidity and invasiveness in TGFβ-stimulated NSCLC cells. Our experimental results provide insights into TGFβ-induced cytoskeletal remodeling of NSCLC cells and suggest that mediators of elevated cell stiffness and migratory activity such as unconventional cytoskeletal and motor proteins may represent promising pharmaceutical targets for restraining invasive spread of lung cancer.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

