Epigallocatechin-3-gallate enhances the osteoblastogenic differentiation of human adipose-derived stem cells
- PMID: 31114166
- PMCID: PMC6485322
- DOI: 10.2147/DDDT.S192683
Epigallocatechin-3-gallate enhances the osteoblastogenic differentiation of human adipose-derived stem cells
Abstract
Purpose: The aim of this study is to investigate the effects of epigallocatechin-3-gallate (EGCG), a major polyphenol extracted from green tea, on the osteoblastogenic differentiation of human adipose-derived stem cells (hASCs).
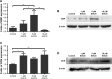
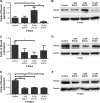
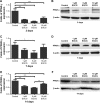
Patients and methods: hASCs were acquired from human adipose tissue. With informed consent, subcutaneous adipose tissue samples were harvested from periorbital fat pad resections from ten healthy female adults who underwent double eyelid surgery. hASCs were cultured in osteogenic medium with or without EGCG (1 μM, 5 μM, or 10 μM) for 14 days. We evaluated the effects of EGCG by quantifying cell growth, ALP activity (an early osteoblastogenic differentiation marker), BSP, OCN (a late osteoblastogenic differentiation marker), and extracellular matrix mineralization. We also performed Western blots to measure osteoblastogenesis-related proteins such as Runx2 and adipoblastogenesis-related transcription factors, such as STAT3, C/EBP-α, and PPAR-γ.
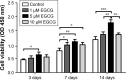
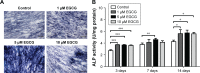
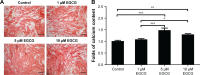
Results: EGCG at 5 μM resulted in significantly higher cell proliferation and ALP activity than did the control on days 3, 7, and 14. On day 7, 5 μM EGCG significantly enhanced BSP expression. On day 14, EGCG at all concentrations promoted OCN expression. In addition, EGCG at 5 μM resulted in the highest level of extracellular matrix mineralization. On day 3, the expression levels of Runx2 were significantly higher in the 5 μM EGCG group than in the other groups, whereas later, on days 7 and 14, Runx2 expression levels in the EGCG group were significantly lower than those of the control group. EGCG at all three concentrations was associated with significantly lower levels of phosphorylated STAT3, C/EBP-α, and PPAR-γ.
Conclusion: EGCG at 5 μM significantly enhanced the osteoblastogenic differentiation of hASCs.
Keywords: EGCG; STAT3; bone regeneration; hASCs; osteoblastogenesis.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


References
-
- Calori GM, Albisetti W, Agus A, Iori S, Tagliabue L. Risk factors contributing to fracture non-unions. Injury. 2007;38(Suppl 2):S11–S18. - PubMed
-
- Einhorn TA. Enhancement of fracture-healing. J Bone Joint Surg Am. 1995;77(6):940–956. - PubMed
-
- Tzioupis C, Giannoudis PV. Prevalence of long-bone non-unions. Injury. 2007;38(Suppl 2):S3–S9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

