SLC5A1 promotes growth and proliferation of pancreatic carcinoma via glucose-dependent AMPK/mTOR signaling
- PMID: 31114359
- PMCID: PMC6489640
- DOI: 10.2147/CMAR.S195424
SLC5A1 promotes growth and proliferation of pancreatic carcinoma via glucose-dependent AMPK/mTOR signaling
Abstract
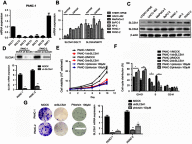
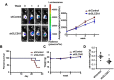
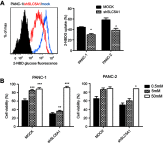
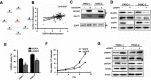
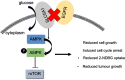
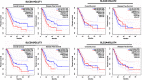
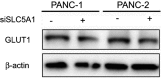
Background: Accumulating studies have reported that aberrant expression of SLC5A1 is a negative prognostic factor to various cancer patients. Purpose: Pancreatic cancer tissue has also shown to harbor higher expression of SLC5A1, however how SLC5A1 mediates pancreatic cancer cells growth remains unclear. Methods: In this study, we examined the mRNA and protein expressions of SLC5A1 in human pancreatic tissue and various cell lines. The in vitro and in vivo roles of SLC5A1 in pancreatic cancer were investigated through stably transfected pancreatic cells with shRNA plasmid targeting SLC5A1. Results: Our results observed SLC5A1 was over-expressed in human pancreatic cancer tissues as well as most pancreatic cancer cell lines. Both in vitro and in vivo inhibition of SLC5A1 retarded pancreatic cancer cell growth and progression. The SLC5A1 knockdown mediated growth suppression is mainly regulated by reduced cellular glucose uptake by pancreatic cancer cells. Our further mechanistic observation showed that inhibition of SLC5A1 induced AMPK-dependent mTOR suppression and pharmacological inhibition of AMPK rescued the effect of SLC5A1 blockade. Further protein-protein interaction analysis showed association of SLC5A1 with EGFR and knockdown of EGFR also showed decreased cellular survival and glucose uptake by pancreatic cancer cells. Conclusion: Our findings postulated SLC5A1/EGFR as the potential therapeutic target of pancreatic cancer patients.
Keywords: EGFR; SLC5A1; cancer cell survival; pancreatic cancer.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

