The JAK/STAT Pathway in Skeletal Muscle Pathophysiology
- PMID: 31114509
- PMCID: PMC6502894
- DOI: 10.3389/fphys.2019.00500
The JAK/STAT Pathway in Skeletal Muscle Pathophysiology
Abstract
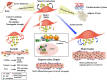
The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway is a key intracellular mediator of a variety of metabolically relevant hormones and cytokines, including the interleukin-6 (IL-6) family of cytokines. The JAK/STAT pathway transmits extracellular signals to the nucleus, leading to the transcription of genes involved in multiple biological activities. The JAK/STAT pathway has been reported to be required for the homeostasis of different tissues and organs. Indeed, when deregulated, it promotes the initiation and progression of pathological conditions, including cancer, obesity, diabetes, and other metabolic diseases. In skeletal muscle, activation of the JAK/STAT pathway by the IL-6 cytokines accounts for opposite effects: on the one hand, it promotes muscle hypertrophy, by increasing the proliferation of satellite cells; on the other hand, it contributes to muscle wasting. The expression of IL-6 and of key members of the JAK/STAT pathway is regulated at the epigenetic level through histone methylation and histone acetylation mechanisms. Thus, manipulation of the JAK/STAT signaling pathway by specific inhibitors and/or drugs that modulate epigenetics is a promising therapeutic intervention for the treatment of numerous diseases. We focus this review on the JAK/STAT pathway functions in striated muscle pathophysiology and the potential role of IL-6 as an effector of the cross talk between skeletal muscle and other organs.
Keywords: IL-6 cytokine; JAK/STAT pathway; epigenetics; organ cross talk; skeletal muscle.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

