The Phenotypic Analysis of Lactobacillus plantarum shsp Mutants Reveals a Potential Role for hsp1 in Cryotolerance
- PMID: 31114549
- PMCID: PMC6503756
- DOI: 10.3389/fmicb.2019.00838
The Phenotypic Analysis of Lactobacillus plantarum shsp Mutants Reveals a Potential Role for hsp1 in Cryotolerance
Abstract
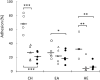
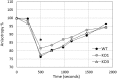
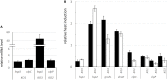
Small heat shock proteins (sHSPs) are ubiquitous, low molecular weight (MW) proteins that share a conserved alpha-crystallin domain. sHSPs oligomers exhibit chaperon-like activities by interacting with unfolded substrates, thereby preventing their aggregation and precipitation. Unlike most lactobacilli, which have single shsp genes, three different sHSP-encoding genes, i.e., hsp1, hsp2, and hsp3, were previously identified in the probiotic Lactobacillus plantarum WCFS1. Early studies, including the characterization of the knock out (KO) mutant for hsp2, indicated a different organization and transcriptional regulation of these genes and suggested that the three L. plantarum sHSPs might accomplish different tasks in stress response. To unravel the role of sHSPs, KO mutants of hsp1 and hsp3 were generated using a Cre-lox based system. Mutation of either genes resulted in impaired growth capacity under normal conditions, heat-stress and stresses typically found during host interactions and food technological process. However, survival to heat shock and the level of thermal stabilization of cytoplasmic proteins were similar between mutants and parental strain. Transcriptional analysis revealed that in the mutant genetic backgrounds there is an upregulated basal expression of the un-mutated mate hsps and other stress-related genes, which may compensate for the loss of HSP function, hence possibly accounting for the lack of a remarkable susceptibility to heat challenge. HSP3 seemed relevant for the induction of thermotolerance, while HSP1 was required for improved cryotolerance. Cell surface properties and plasma membrane fluidity were investigated to ascertain the possible membrane association of sHSP. Intriguingly, the loss of hsp1 was associated to a lower level of maximal membrane fluidity upon heat stress. A role for HSP1 in controlling and improving membrane fluidity is suggested which may pertains its cryoprotective function.
Keywords: Lactobacillus plantarum; chaperone; membrane fluidity; probiotic; small heat shock proteins (sHSP); stress.
Figures

References
-
- Arena M. P., Romano A., Capozzi V., Beneduce L., Ghariani M., Grieco F., et al. (2011). Expression of Lactobacillus brevis IOEB 9809 tyrosine decarboxylase and agmatine deiminase genes in wine correlates with substrate availability. Lett. Appl. Microbiol. 53 395–402. 10.1111/j.1472-765X.2011.03120.x - DOI - PubMed
-
- Beal C., Fonseca F. (2015). “Freezing of probiotic bacteria,” in Advances in Probiotic Technology, eds Foerst P., Santivarangkna C. (Boca Raton, FL: CRC Press; ), 179–212. 10.1201/b18807-14 - DOI
-
- Bellon-Fontaine M. N., Rault J., Van Oss C. J. (1996). Microbial adhesion to solvents: a novel method to determine the electron–donor/electron–acceptor or Lewis acid–base properties of microbial-cells. Colloids Surf. 7 47–53. 10.1016/0927-7765(96)01272-6 - DOI
-
- Bove P., Capozzi V., Fiocco D., Spano G. (2011). Involvement of the sigma factor sigma H in the regulation of a small heat shock protein gene in Lactobacillus plantarum WCFS1. Ann. Microbiol. 61 973–977. 10.1007/s13213-011-0283-9 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

