Challenge to the Intestinal Mucosa During Sepsis
- PMID: 31114571
- PMCID: PMC6502990
- DOI: 10.3389/fimmu.2019.00891
Challenge to the Intestinal Mucosa During Sepsis
Abstract
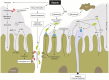
Sepsis is a complex of life-threating organ dysfunction in critically ill patients, with a primary infectious cause or through secondary infection of damaged tissues. The systemic consequences of sepsis have been intensively examined and evidences of local alterations and repercussions in the intestinal mucosal compartment is gradually defining gut-associated changes during sepsis. In the present review, we focus on sepsis-induced dysfunction of the intestinal barrier, consisting of an increased permeability of the epithelial lining, which may facilitate bacterial translocation. We discuss disturbances in intestinal vascular tonus and perfusion and coagulopathies with respect to their proposed underlying molecular mechanisms. The consequences of enzymatic responses by pancreatic proteases, intestinal alkaline phosphatases, and several matrix metalloproteases are also described. We conclude our insight with a discussion on novel therapeutic interventions derived from crucial aspects of the gut mucosal dynamics during sepsis.
Keywords: enzymatic response; gut-barrier dysfunction; innate immunity; microbiome; perfusion disturbances; sepsis.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

