TRAIL-R1 and TRAIL-R2 Mediate TRAIL-Dependent Apoptosis in Activated Primary Human B Lymphocytes
- PMID: 31114586
- PMCID: PMC6503035
- DOI: 10.3389/fimmu.2019.00951
TRAIL-R1 and TRAIL-R2 Mediate TRAIL-Dependent Apoptosis in Activated Primary Human B Lymphocytes
Abstract
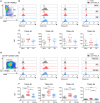
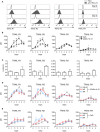
The maintenance of B cell homeostasis requires a tight control of B cell generation, survival, activation, and maturation. In lymphocytes upon activation, increased sensitivity to apoptotic signals helps controlling differentiation and proliferation. The death receptor Fas is important in this context because genetic Fas mutations in humans lead to an autoimmune lymphoproliferative syndrome that is similar to lymphoproliferation observed in Fas-deficient mice. In contrast, the physiological role of TNF-related apoptosis-inducing ligand receptors (TRAIL-Rs) in humans has been poorly studied so far. Indeed, most studies have focused on tumor cell lines and on mouse models whose results are difficult to transpose to primary human B cells. In the present work, the expression of apoptosis-inducing TRAIL-R1 and TRAIL-R2 and of the decoy receptors TRAIL-R3 and TRAIL-R4 was systematically studied in all developmental stages of peripheral B cells isolated from the blood and secondary lymphoid organs. Expression of TRAIL-Rs is modulated along development, with highest levels observed in germinal center B cells. In addition, T-dependent and T-independent signals elicited induction of TRAIL-Rs with distinct kinetics, which differed among B cell subpopulations: switched memory cells rapidly upregulated TRAIL-R1 and -2 upon activation while naïve B cells only reached similar expression levels at later time points in culture. Increased expression of TRAIL-R1 and -2 coincided with a caspase-3-dependent sensitivity to TRAIL-induced apoptosis in activated B cells but not in freshly isolated resting B cells. Finally, both TRAIL-R1 and TRAIL-R2 could signal actively and both contributed to TRAIL-induced apoptosis. In conclusion, this study provides a systematic analysis of the expression of TRAIL-Rs in human primary B cells and of their capacity to signal and induce apoptosis. This dataset forms a basis to further study and understand the dysregulation of TRAIL-Rs and TRAIL expression observed in autoimmune diseases. Additionally, it will be important to foresee potential bystander immunomodulation when TRAIL-R agonists are used in cancer treatment.
Keywords: B lymphocytes; TRAIL; TRAIL-R; apoptosis; human.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

