DNA specificities modulate the binding of human transcription factor A to mitochondrial DNA control region
- PMID: 31114891
- PMCID: PMC6614842
- DOI: 10.1093/nar/gkz406
DNA specificities modulate the binding of human transcription factor A to mitochondrial DNA control region
Abstract
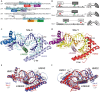
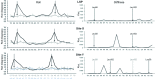
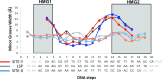
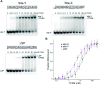
Human mitochondrial DNA (h-mtDNA) codes for 13 subunits of the oxidative phosphorylation pathway, the essential route that produces ATP. H-mtDNA transcription and replication depends on the transcription factor TFAM, which also maintains and compacts this genome. It is well-established that TFAM activates the mtDNA promoters LSP and HSP1 at the mtDNA control region where DNA regulatory elements cluster. Previous studies identified still uncharacterized, additional binding sites at the control region downstream from and slightly similar to LSP, namely sequences X and Y (Site-X and Site-Y) (Fisher et al., Cell 50, pp 247-258, 1987). Here, we explore TFAM binding at these two sites and compare them to LSP by multiple experimental and in silico methods. Our results show that TFAM binding is strongly modulated by the sequence-dependent properties of Site-X, Site-Y and LSP. The high binding versatility of Site-Y or the considerable stiffness of Site-X tune TFAM interactions. In addition, we show that increase in TFAM/DNA complex concentration induces multimerization, which at a very high concentration triggers disruption of preformed complexes. Therefore, our results suggest that mtDNA sequences induce non-uniform TFAM binding and, consequently, direct an uneven distribution of TFAM aggregation sites during the essential process of mtDNA compaction.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


Similar articles
-
Cytosine methylation of mitochondrial DNA at CpG sequences impacts transcription factor A DNA binding and transcription.Biochim Biophys Acta Gene Regul Mech. 2019 May;1862(5):598-607. doi: 10.1016/j.bbagrm.2019.01.006. Epub 2019 Feb 23. Biochim Biophys Acta Gene Regul Mech. 2019. PMID: 30807854 Free PMC article.
-
Distinct structural features of TFAM drive mitochondrial DNA packaging versus transcriptional activation.Nat Commun. 2014;5:3077. doi: 10.1038/ncomms4077. Nat Commun. 2014. PMID: 24435062 Free PMC article.
-
U-turn DNA bending by human mitochondrial transcription factor A.Curr Opin Struct Biol. 2013 Feb;23(1):116-24. doi: 10.1016/j.sbi.2012.12.004. Epub 2013 Jan 16. Curr Opin Struct Biol. 2013. PMID: 23333034 Review.
-
Mitochondrial transcription factor A promotes DNA strand cleavage at abasic sites.Proc Natl Acad Sci U S A. 2019 Sep 3;116(36):17792-17799. doi: 10.1073/pnas.1911252116. Epub 2019 Aug 14. Proc Natl Acad Sci U S A. 2019. PMID: 31413200 Free PMC article.
-
Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number.Biochim Biophys Acta. 2012 Sep-Oct;1819(9-10):921-9. doi: 10.1016/j.bbagrm.2012.03.002. Epub 2012 Mar 21. Biochim Biophys Acta. 2012. PMID: 22465614 Review.
Cited by
-
DNA-protein cross-links between abasic DNA damage and mitochondrial transcription factor A (TFAM).Nucleic Acids Res. 2023 Jan 11;51(1):41-53. doi: 10.1093/nar/gkac1214. Nucleic Acids Res. 2023. PMID: 36583367 Free PMC article.
-
Characterization of the Complete Mitochondrial Genome of the Central Highland Grey-Shanked Douc Langur (Pygathrix cinerea), a Critically Endangered Species Endemic to Vietnam (Mammalia: Primates).Curr Issues Mol Biol. 2024 Sep 6;46(9):9928-9947. doi: 10.3390/cimb46090592. Curr Issues Mol Biol. 2024. PMID: 39329944 Free PMC article.
-
A minimal motif for sequence recognition by mitochondrial transcription factor A (TFAM).Nucleic Acids Res. 2022 Jan 11;50(1):322-332. doi: 10.1093/nar/gkab1230. Nucleic Acids Res. 2022. PMID: 34928349 Free PMC article.
-
Structural analysis of the Candida albicans mitochondrial DNA maintenance factor Gcf1p reveals a dynamic DNA-bridging mechanism.Nucleic Acids Res. 2023 Jun 23;51(11):5864-5882. doi: 10.1093/nar/gkad397. Nucleic Acids Res. 2023. PMID: 37207342 Free PMC article.
-
Comparative mitogenome analyses uncover mitogenome features and phylogenetic implications of the subfamily Cobitinae.BMC Genomics. 2021 Jan 14;22(1):50. doi: 10.1186/s12864-020-07360-w. BMC Genomics. 2021. PMID: 33446100 Free PMC article.
References
-
- Lopez-Lluch G. Mitochondrial activity and dynamics changes regarding metabolism in ageing and obesity. Mech. Ageing. Dev. 2017; 162:108–121. - PubMed
-
- Burte F., Carelli V., Chinnery P.F., Yu-Wai-Man P.. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat. Rev. Neurol. 2015; 11:11–24. - PubMed
-
- Chang D.D., Clayton D.A.. Precise identification of individual promoters for transcription of each strand of human mitochondrial DNA. Cell. 1984; 36:635–643. - PubMed

