Induction of ethylene inhibits development of soybean sudden death syndrome by inducing defense-related genes and reducing Fusarium virguliforme growth
- PMID: 31116746
- PMCID: PMC6530837
- DOI: 10.1371/journal.pone.0215653
Induction of ethylene inhibits development of soybean sudden death syndrome by inducing defense-related genes and reducing Fusarium virguliforme growth
Abstract
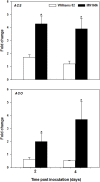
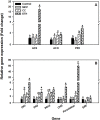
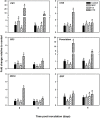
Ethylene is a gaseous hormone that regulates plant responses to biotic and abiotic stresses. To investigate the importance of ethylene in soybean resistance to Fusarium virguliforme (Fv), the causal agent of sudden death syndrome (SDS), soybean cultivars Williams 82 (SDS-susceptible) and MN1606 (SDS-resistant) were treated 24 h before and 24h after Fv inoculation with either ethephon (ethylene inducer), cobalt chloride (ethylene biosynthesis inhibitor), or 1-MCP (ethylene perception inhibitor). Inoculated plants were grown for 21 days at 24°C in the greenhouse and then evaluated for SDS severity and expression of soybean defense genes. In both cultivars, plants treated with ethephon showed lower SDS foliar severity compared to the other treatments, whereas those treated with cobalt chloride or 1-MCP showed the same or higher SDS foliar severity compared to the water-treated control. Ethephon application resulted in activation of genes involved in ethylene biosynthesis, such as ethylene synthase (ACS) and ethylene oxidase (ACO), and genes involved in soybean defense response, such as pathogenesis-related protein (PR), basic peroxidase (IPER), chalcone synthase (CHS), and defense-associated transcription factors. Cobalt chloride and 1-MCP treatments had little or no effect on the expression of these genes. In addition, ethephon had a direct inhibitory effect on in-vitro growth of Fv on PDA media. Our results suggest that ethephon application inhibits SDS development directly by slowing Fv growth and/or by inducing soybean ethylene signaling and the expression of defense related genes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Aoki T, O’Donnell K, Scandiani MM. Sudden death syndrome of soybean in South America is caused by four species of Fusarium: Fusarium brasiliense sp. nov., F. cuneirostrum sp. nov., F. tucumaniae, and F. virguliforme. Mycoscience. 2005;46: 162–183. 10.1007/s10267-005-0235-y - DOI
-
- Leandro LFS, Robertson AE, Mueller DS. Climatic and Environmental Trends Observed During Epidemic and Non-epidemic Years of Soybean Sudden Death Syndrome in Iowa Plant Health Progress Plant Health Progress. Plant Manag Netw. 2013; 10.1094/PHP-2013-0529-01-RS.Abstract - DOI
-
- Leandro LF, Tatalovic N, Luckew A. Soybean sudden death syndrome—advances in knowledge and disease management. CAB Rev. 2012; 7(53): 1–14. 10.1079/PAVSNNR20127053 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

