Losartan and isoproterenol promote alterations in the local renin-angiotensin system of rat salivary glands
- PMID: 31116771
- PMCID: PMC6530859
- DOI: 10.1371/journal.pone.0217030
Losartan and isoproterenol promote alterations in the local renin-angiotensin system of rat salivary glands
Abstract
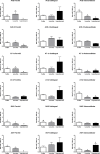
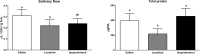
Renin-angiotensin system (RAS) systemically or locally collaborates with tissue homeostasis, growth and development, which has been extensively studied for its pharmacological implications. This study was primarily aimed at finding and characterizing local RAS in rat parotid, sublingual and submandibular glands. It was also hypothesized that vasoactive drugs could affect the expression of RAS targets, as well as saliva flow and its composition. Therefore, another objective of this study was to compare the effects of losartan (angiotensin II receptor blocker) and isoproterenol (β-adrenergic receptor agonist). Forty-one Wistar rats were divided into three groups and administered a daily intraperitoneal dose of saline, losartan or isoproterenol solutions for one week. The following RAS targets were studied using qPCR: renin (REN), angiotensinogen (AGT), angiotensin converting enzyme (ACE), ACE-2, elastase-2 (ELA-2), AT1-a and MAS receptors, using RPL-13 as a reference gene. Morphology of glands was analyzed by immunohistochemistry using REN, ACE, ACE-2, AT1, AT2 and MAS antibodies. The volume and total protein content of saliva were measured. Our results revealed that ACE, ACE-2, AT1-a, AT2 and MAS receptors were expressed in all salivary gland samples, but REN and ELA-2 were absent. Losartan decreased mRNA expression of RAS targets in parotid (MAS) and submandibular glands (ACE and both AT receptors), without affecting morphological alterations, and significantly decreased saliva and total protein secretions. Isoproterenol treatment affected gene expression profiles in parotid (ACE, ACE-2, AT1-a, MAS, AGT), and submandibular (ACE, AT2, AGT) glands, thus promoting acinar hypertrophy in serous acini, without significant changes in salivary flow or total protein content. These drugs affected mainly acini, followed by duct systems and myoepithelial cells, whereas blood vessels were not affected. In conclusion, there is a local RAS in major rat salivary glands and losartan, an angiotensin II receptor blocker, affected not only the RAS-target gene expression but also decreased salivary flow and total protein content.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Hall JE. O Papel dos Rins no Controle a Longo Prazo da Pressão Arterial e Na Hipertensão: O Sistema Integrado De Regulação da Pressão Arterial. Guyton e Hall: Tratado de Fisiologia Médica. 13th ed Rio de Janeiro: Elsevier Editora Ltda; 2017. pp. 227–41.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

