Efficacy between low and high dose aspirin for the initial treatment of Kawasaki disease: Current evidence based on a meta-analysis
- PMID: 31117119
- PMCID: PMC6531010
- DOI: 10.1371/journal.pone.0217274
Efficacy between low and high dose aspirin for the initial treatment of Kawasaki disease: Current evidence based on a meta-analysis
Abstract
Background: Kawasaki disease (KD) is now the leading cause of acquired heart disease in children in developed countries. Intravenous immunoglobulin (IVIG) and aspirin were considered as the standard initial treatment of KD for decades. However, the optimal dose of aspirin has remained controversial. In recent years, many studies compared the efficacy of low-dose with high-dose aspirin in the acute phase of KD, but the results have not always been consistent. Therefore, we performed this meta-analysis to evaluate the efficacy of low-dose aspirin compared with high-dose for the initial treatment of KD.
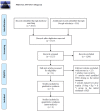
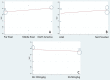
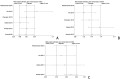
Methods: Studies related to aspirin therapy for KD were selected from PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, China National Knowledge Infrastructure, and Google scholar through Mar 25th, 2019. Data were analyzed using STATA Version 15.1. Additionally, publication bias and sensitivity analysis were also performed by STATA version 15.1.
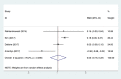
Results: Six studies were included in our analysis of the rate of coronary artery lesion (CAL), five reports for IVIG-resistant KD (rKD), and four for the duration of fever and hospitalization. However, no significant differences were found between low-dose and high-dose aspirin groups in the incidence of CAL (risk ratio (RR), 0.85; 95%CI (0.63, 1.14); P = 0.28), the risk of rKD (RR, 1.39; 95%CI (1.00, 1.93); P = 0.05), and duration of fever and hospitalization (the mean standard deviation (SMD), 0.03; 95%CI (-0.16, 0.22); P = 0.78).
Conclusion: Low-dose aspirin (3-5 mg·kg-1·d-1) may be as effective as the use of high-dose aspirin (≥30 mg·kg-1·d-1) for the initial treatment of KD. Further well-designed randomized clinical trials are needed to evaluate the efficacy of low-dose aspirin for the initial treatment of KD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747–2771. 10.1161/01.CIR.0000145143.19711.78 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

