Metabolite Responsive Nanoparticle-Protein Complex
- PMID: 31117354
- PMCID: PMC7819679
- DOI: 10.1021/acs.biomac.9b00470
Metabolite Responsive Nanoparticle-Protein Complex
Abstract
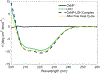
Stimuli-responsive polymers are an efficient means of targeted therapy. Compared to conventional agents, they increase bioavailability and efficacy. In particular, polymer hydrogel nanoparticles (NPs) can be designed to respond when exposed to a specific environmental stimulus such as pH or temperature. However, targeting a specific metabolite as the trigger for stimuli response could further elevate selectivity and create a new class of bioresponsive materials. In this work we describe an N-isopropylacrylamide (NIPAm) NP that responds to a specific metabolite, characteristic of a hypoxic environment found in cancerous tumors. NIPAm NPs were synthesized by copolymerization with an oxamate derivative, a known inhibitor of lactate dehydrogenase (LDH). The oxamate-functionalized NPs (OxNP) efficiently sequestered LDH to produce an OxNP-protein complex. When exposed to elevated concentrations of lactic acid, a substrate of LDH and a metabolite characteristic of hypoxic tumor microenvironments, OxNP-LDH complexes swelled (65%). The OxNP-LDH complexes were not responsive to structurally related small molecules. This work demonstrates a proof of concept for tuning NP responsiveness by conjugation with a key protein to target a specific metabolite of disease.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Kabanov AV; Bronich TK; Kabanov VA; Yu K; Eisenberg A Soluble stoichiometric complexes from poly(N-ethyl-4-vinylpyridinium) cations and poly(ethylene oxide)-block-polymethacrylate anions. Macromolecules 1996, 29, 6797–6802.
-
- Harada A; Kataoka K Formation of polyion complex micelles in an aqueous milieu from a pair of oppositely-charged block copolymers with poly(ethylene glycol) segments. Macromolecules 1995, 28, 5294–5299.
-
- Bae Y; Fukushima S; Harada A; Kataoka K Design of environment-sensitive Supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew. Chem., Int. Ed. 2003, 42, 4640–4643. - PubMed
-
- Lee Y; Fukushima S; Bae Y; Hiki S; Ishii T; Kataoka K A protein nanocarrier from chargeconversion polymer in response to endosomal pH. J. Am. Chem. Soc. 2007, 129, 5362–5363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

