HAI and NAI titer correlates of inactivated and live attenuated influenza vaccine efficacy
- PMID: 31117986
- PMCID: PMC6530189
- DOI: 10.1186/s12879-019-4049-5
HAI and NAI titer correlates of inactivated and live attenuated influenza vaccine efficacy
Abstract
Background: High hemagglutination inhibition (HAI) and neuraminidase inhibition (NAI) titers are generally associated with reduced influenza risk. While repeated influenza vaccination reduces seroresponse, vaccine effectiveness is not always reduced.
Methods: During the 2007-2008 influenza season, a randomized, placebo-controlled trial (FLUVACS) evaluated the efficacies of live-attenuated (LAIV) and inactivated influenza vaccines (IIV) among healthy adults aged 18-49 in Michigan; IIV vaccine efficacy (VE) and LAIV VE against influenza disease were estimated at 68% and 36%. Using the principal stratification/VE moderation framework, we analyzed data from this trial to assess how each VE varied by HAI or NAI responses to vaccination observed for vaccinated individuals and predicted counterfactually for placebo recipients. We also assessed how each VE varied with pre-vaccination/baseline variables including HAI titer, NAI titer, and vaccination history.
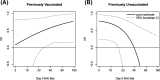
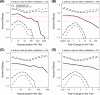
Results: IIV VE appeared to increase with Day 30 post-vaccination HAI titer, albeit not significantly (p=0.20 and estimated VE 14.4%, 70.5%, and 85.5% at titer below the assay lower quantification limit, 512, and 4096 (maximum)). Moreover, IIV VE increased significantly with Day 30 post-vaccination NAI titer (p=0.040), with estimated VE zero at titer 10 and 92.2% at highest titer 640. There was no evidence that fold-change in post-vaccination HAI or NAI titer associated with IIV VE (p=0.76, 0.38). For LAIV, there was no evidence that VE associated with post-vaccination or fold-rise HAI or NAI titers (p-values >0.40). For IIV, VE increased with increasing baseline NAI titer in those previously vaccinated, but VE decreased with increasing baseline NAI titer in those previously unvaccinated. In contrast, for LAIV, VE did not depend on previous vaccination or baseline HAI or NAI titer.
Conclusions: Future efficacy trials should measure baseline and post-vaccination antibody titers in both vaccine and control/placebo recipients, enabling analyses to better elucidate correlates of vaccine- and natural-protection.
Trial registration: ClinicalTrials.gov NCT00538512. October 1, 2007.
Keywords: FLUVACS trial; Hemagglutinin inhibition (HAI) titers; Immune correlates; Neuraminidase inhibition (NAI) titer; Principal stratification/vaccine efficacy moderation framework; Vaccine efficacy.
Conflict of interest statement
Arnold S. Monto declares grant support from Sanofi Pasteur and consultancy fees from Sanofi Pasteur, Novartis, and Novavax. Peter B. Gilbert declares grant support from Sanofi Pasteur.
Figures




References
-
- Coudeville L, Bailleux F, Riche B, Megas F, Andre P, Ecochard R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: Development and application of a bayesian random-effects model. BMC Med Res Methodol. 2010;10:18. doi: 10.1186/1471-2288-10-18. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

