The PRINTO evidence-based proposal for glucocorticoids tapering/discontinuation in new onset juvenile dermatomyositis patients
- PMID: 31118099
- PMCID: PMC6530070
- DOI: 10.1186/s12969-019-0326-5
The PRINTO evidence-based proposal for glucocorticoids tapering/discontinuation in new onset juvenile dermatomyositis patients
Abstract
Background: Prednisone (PDN) in juvenile dermatomyositis (JDM), alone or in association with other immunosuppressive drugs, namely methotrexate (MTX) and cyclosporine (CSA), represents the first-line treatment option for new onset JDM patients. No clear evidence based guidelines are actually available to standardize the tapering and discontinuation of glucocorticoids (GC) in JDM. Aim of our study was to provide an evidence-based proposal for GC tapering/discontinuation in new onset juvenile dermatomyositis (JDM), and to identify predictors of clinical remission and GC discontinuation.
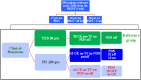
Methods: New onset JDM children were randomized to receive either PDN alone or in combination with methotrexate (MTX) or cyclosporine (CSA). In order to derive steroid tapering indications, PRINTO/ACR/EULAR JDM core set measures (CSM) and their median absolute and relative percent changes over time were compared in 3 groups. Group 1 included those in clinical remission who discontinued PDN, with no major therapeutic changes (MTC) (reference group) and was compared with those who did not achieve clinical remission, without or with MTC (Group 2 and 3, respectively). A logistic regression model identified predictors of clinical remission with PDN discontinuation.
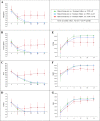
Results: Based on the median change in the CSM of 30/139 children in Group 1, after 3 pulses of methyl-prednisolone, GC could be tapered from 2 to 1 mg/kg/day in the first two months from onset if any of the CSM decreased by 50-94%, and from 1 to 0.2 mg/kg/day in the following 4 months if any CSM further decreased by 8-68%, followed by discontinuation in the ensuing 18 months. The achievement of PRINTO JDM 50-70-90 response after 2 months of treatment (ORs range 4.5-6.9), an age at onset > 9 years (OR 4.6) and the combination therapy PDN + MTX (OR 3.6) increase the probability of achieving clinical remission (p < 0.05).
Conclusions: This is the first evidence-based proposal for glucocorticoid tapering/discontinuation based on the change in JDM CSM of disease activity.
Trial registration: Trial full title: Five-Year Single-Blind, Phase III Effectiveness Randomized Actively Controlled Clinical Trial in New Onset Juvenile Dermatomyositis: Prednisone versus Prednisone plus Cyclosporine A versus Prednisone plus Methotrexate. EUDRACT registration number: 2005-003956-37 .
Clinical trial: gov is NCT00323960 . Registered on 17 August 2005.
Keywords: Core set measures; Disease activity; Glucorticoids; Juvenile dermatomyositis; Prednisone tapering.
Conflict of interest statement
Gabriella Giancane has no conflicts of interest to disclose.
Claudio Lavarello has no conflicts of interest to disclose.
Angela Pistorio has no conflicts of interest to disclose.
Sheila K. Oliveira has no conflicts of interest to disclose.
Francesco Zulian has no conflicts of interest to disclose.
Ruben Cuttica reports speaker bureau, consulting and Principal Investigator for Abbvie, BMS, Centocor, GSK, Lilly, Pfizer, Roche, Sanofi, UCB (< 10.000 USD each).
Michel Fischbach has no conflicts of interest to disclose.
Bo Magnusson has no conflicts of interest to disclose.
Serena Pastore has no conflicts of interest to disclose.
Roberto Marini has no conflicts of interest to disclose.
Silvana Martino has no conflicts of interest to disclose.
Anne Pagnier has no conflicts of interest to disclose.
Christine Soler has no conflicts of interest to disclose.
ValdaStaņēvicha has no conflicts of interest to disclose.
Rebecca Ten Cate has no conflicts of interest to disclose.
Yosef Uziel has received speaker’s bureaus or consulting fees from: AbbVie, Pfizer, Novartis (< 10.000 USD each).
JelenaVojinovic has no conflicts of interest to disclose.
Elena Fueri has no conflicts of interest to disclose.
Angelo Ravelli has received speaker’s bureaus or consulting fees from: AbbVie, BMS, Pfizer,
Hoffman LaRoche, Novartis, Centocor (< 10.000 USD each).
Alberto Martini does not have any conflict of interest to declare since March 2016, when he became the Scientific Director of the Gaslini Institute because this role does not allow him to render private consultancy resulting in personal income. AM performed consultancy activities on behalf of the Gaslini Institute for AbbVie, Boehringer, Novartis, and R-Pharm (< 10.000 USD each).
Nicolino Ruperto has received honoraria for consultancies or speaker bureaus (< 10.000 USD each) from the following pharmaceutical companies in the past 3 years: Ablynx, AbbVie, Astrazeneca-Medimmune, Biogen, Boehringer, Bristol Myers and Squibb, Eli-Lilly, EMD Serono, Glaxo Smith and Kline, Hoffmann-La Roche, Janssen, Merck, Novartis, Pfizer, R-Pharma, SanofiServier, Sinergie, Sobi and Takeda.
The Gaslini Hospital, where NR works as full-time public employee, has received contributions (> 10.000 USD each) from the following industries in the last 3 years: BMS, Eli-Lilly, GlaxoSmithKline, F Hoffmann-La Roche, Janssen, Novartis, Pfizer, Sobi. This funding has been reinvested for the research activities of the hospital in a fully independent manner, without any commitment with third parties.
Figures

References
-
- Hasija R, Pistorio A, Ravelli A, Demirkaya E, Khubchandani R, Guseinova D, et al. Therapeutic Approaches in the Treatment of Juvenile Dermatomyositis in Patients With Recent-Onset Disease and in Those Experiencing Disease Flare. Arthritis Rheum 2011;(63):3142–52.Arthritis Rheum 2011 Jun 6;63(10):3142–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

