Efficacy of the mermithid nematode, Romanomermis iyengari, for the biocontrol of Anopheles gambiae, the major malaria vector in sub-Saharan Africa
- PMID: 31118105
- PMCID: PMC6530168
- DOI: 10.1186/s13071-019-3508-6
Efficacy of the mermithid nematode, Romanomermis iyengari, for the biocontrol of Anopheles gambiae, the major malaria vector in sub-Saharan Africa
Abstract
Background: The intensive use of chemical insecticides against mosquitoes has led to the development of widespread insecticide resistance. Control of Anopheles mosquitoes in malaria endemic areas of sub-Saharan Africa has become increasingly difficult. There is an urgent need for malaria control programmes to adopt more integrated mosquito management approaches that include sustainable, nonchemical solutions. The mermithid nematode Romanomermis iyengari is one of several natural control alternatives to synthetic pesticides for mosquito suppression. This study evaluated the effectiveness of the nematode R. iyengari for control of Anopheles gambiae.
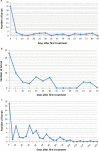
Methods: The nematode R. iyengari was mass-produced, and pre-parasitic stage (J2) were used for laboratory and field experiments. In laboratory experiments, two concentrations of pre-parasitics (5 and 10 J2 per larva) were tested against first- (L1), second- (L2) and third-instar (L3) larvae of An. gambiae. Infected larvae were observed daily to determine their mortality rate and the number of post-parasitic nematodes emerging from dead larvae. In field experiments, 3500, 4000 and 5000 J2/m2 were sprayed in separate natural Anopheles breeding sites. After treatment, the larval mosquito density in the breeding sites was assessed every 5-7 days.
Results: Laboratory results showed that larval An. gambiae is susceptible to nematode infection: 100% L1 larvae died within 24 hours post-treatment, and 100% of both L2 and L3 larvae died within 7 days, regardless of nematode concentrations. The average number of post-parasitic nematodes emerging per larva increased with increasing nematode concentration. In field experiments, the monthly applications of 3500 to 5000 pre-parasitic nematodes per m2 eliminated larval mosquito development in Anopheles- and mixed breeding sites. Larval mosquito density dramatically decreased five days after the first treatment in all treated sites and was maintained at a very low level during the whole experimental period. Basically, only early instar larva were detected in treated sites throughout the test period. The average number of post-parasitic nematodes emerging per larva collected in treated sites was 1.45, 2, and 5.7 respectively for sites treated with 3500, 4000, and 5000 J2/m2.
Conclusions: Malaria mosquito larvae is susceptible to R. iyengari infection in West Africa. Parasitism intensity depends on tested nematode concentrations. Monthly application of 3500 J2/ m2 was enough to control effectively larval An. gambiae in wetlands and floodable locations in West Africa.
Keywords: Anopheles gambiae; Entomopathogenic nematodes; Malaria; Romanomermis iyengari; Vector control.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Biocontrol of Culex quinquefasciatus using the insect parasitic nematode, Romanomermis iyengari (Nematoda: Mermithidae).Trop Biomed. 2019 Dec 1;36(4):1003-1013. Trop Biomed. 2019. PMID: 33597470
-
The Romanomermis iyengari parasite for Anopheles pseudopunctipennis suppression in natural habitats in Oaxaca State, Mexico.Rev Panam Salud Publica. 1999 Jan;5(1):23-8. doi: 10.1590/s1020-49891999000100004. Rev Panam Salud Publica. 1999. PMID: 10050611
-
Large-scale production of the malaria vector biocontrol agent Romanomermis iyengari (Nematoda: Mermithidae) in Benin, West Africa.Malariaworld J. 2015 Jan 17;6:1. doi: 10.5281/zenodo.10869973. eCollection 2015. Malariaworld J. 2015. PMID: 38779621 Free PMC article.
-
Direct and indirect effects of predation and parasitism on the Anopheles gambiae mosquito.Parasit Vectors. 2020 Jan 30;13(1):43. doi: 10.1186/s13071-020-3915-8. Parasit Vectors. 2020. PMID: 32000840 Free PMC article. Review.
-
Bacterial larvicides used for malaria vector control in sub-Saharan Africa: review of their effectiveness and operational feasibility.Parasit Vectors. 2019 Aug 30;12(1):426. doi: 10.1186/s13071-019-3683-5. Parasit Vectors. 2019. PMID: 31470885 Free PMC article. Review.
Cited by
-
Revisiting genomes of non-model species with long reads yields new insights into their biology and evolution.Front Genet. 2024 Feb 7;15:1308527. doi: 10.3389/fgene.2024.1308527. eCollection 2024. Front Genet. 2024. PMID: 38384712 Free PMC article.
-
Mosquito-Borne Diseases Emergence/Resurgence and How to Effectively Control It Biologically.Pathogens. 2020 Apr 23;9(4):310. doi: 10.3390/pathogens9040310. Pathogens. 2020. PMID: 32340230 Free PMC article. Review.
-
Prevalence of Four Nematode Species (Mermithidae) in Adult Mosquitoes (Diptera: Culicidae): First Comments Since Infection/Parasitism in Fourth-Instar Larvae.Microorganisms. 2024 Nov 21;12(12):2388. doi: 10.3390/microorganisms12122388. Microorganisms. 2024. PMID: 39770591 Free PMC article.
-
First Record of Mermithidae (Enoplea: Mermithida) Parasitizing Philaenus spumarius (Hemiptera: Aphrophoridae) in Central Italy.J Nematol. 2025 Jun 4;57(1):20250019. doi: 10.2478/jofnem-2025-0019. eCollection 2025 Feb. J Nematol. 2025. PMID: 40469271 Free PMC article.
-
Infective capacity of the commercial nematode Steinernema carpocapsae parasitizing Aedes aegypti mosquito larvae in Yucatán México.Helminthologia. 2024 Dec 12;61(3):254-262. doi: 10.2478/helm-2024-0028. eCollection 2024 Sep. Helminthologia. 2024. PMID: 40709106 Free PMC article.
References
-
- Danis M, Gentilini M. Le paludisme fléau mondial. Rev Prat. 1998;48:254–257. - PubMed
-
- Gachot B, Bruneel F, Behr C. Paludisme pernicieux. Rev Prat. 2001;51:638–643. - PubMed
-
- de Jager C, Aneck-Hahn NH, Bornman MS, Farias P, Spanò M. DDT exposure levels and semen quality of young men from a malaria area in South Africa. Malar J. 2012;11(Suppl. 1):P21. doi: 10.1186/1475-2875-11-S1-P21. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical