The structural unit of melanin in the cell wall of the fungal pathogen Cryptococcus neoformans
- PMID: 31118223
- PMCID: PMC6615676
- DOI: 10.1074/jbc.RA119.008684
The structural unit of melanin in the cell wall of the fungal pathogen Cryptococcus neoformans
Abstract
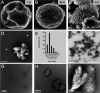
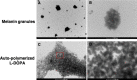
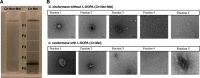
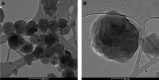
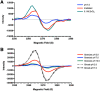
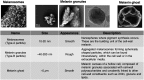
Melanins are synthesized macromolecules that are found in all biological kingdoms. These pigments have a myriad of roles that range from microbial virulence to key components of the innate immune response in invertebrates. Melanins also exhibit unique properties with potential applications in physics and material sciences, ranging from electrical batteries to novel therapeutics. In the fungi, melanins, such as eumelanins, are components of the cell wall that provide protection against biotic and abiotic elements. Elucidation of the smallest fungal cell wall-associated melanin unit that serves as a building block is critical to understand the architecture of these polymers, its interaction with surrounding components, and their functional versatility. In this study, we used isopycnic gradient sedimentation, NMR, EPR, high-resolution microscopy, and proteomics to analyze the melanin in the cell wall of the human pathogenic fungus Cryptococcus neoformans We observed that melanin is assembled into the cryptococcal cell wall in spherical structures ∼200 nm in diameter, termed melanin granules, which are in turn composed of nanospheres ∼30 nm in diameter, termed fungal melanosomes. We noted that melanin granules are closely associated with proteins that may play critical roles in the fungal melanogenesis and the supramolecular structure of this polymer. Using this structural information, we propose a model for C. neoformans' melanization that is similar to the process used in animal melanization and is consistent with the phylogenetic relatedness of the fungal and animal kingdoms.
Keywords: basic unit; biopolymer; cell wall; cryo-electron microscopy; fungi; melanin; melanogenesis; melanosomes; solid-state NMR; supramolecular structure.
© 2019 Camacho et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures







References
-
- Shosuke I., Kazumasa W., Marco d. I, Alessandra N., and Alessandro P. (2011) in Melanins and Melanosomes: Biosynthesis, Biogenesis, Physiological, and Pathological Functions. (Borovansky J., and Patrick A., R., eds) 1st Ed., pp. 167–185, Wiley-VCH Verlag & Co. KGaA, Germany
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

