Schlafen 11 Restricts Flavivirus Replication
- PMID: 31118262
- PMCID: PMC6639263
- DOI: 10.1128/JVI.00104-19
Schlafen 11 Restricts Flavivirus Replication
Abstract
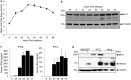
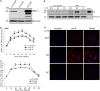
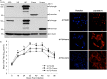
Schlafen 11 (Slfn11) is an interferon-stimulated gene that controls the synthesis of proteins by regulating tRNA abundance. Likely through this mechanism, Slfn11 has previously been shown to impair human immunodeficiency virus type 1 (HIV-1) infection and the expression of codon-biased open reading frames. Because replication of positive-sense single-stranded RNA [(+)ssRNA] viruses requires the immediate translation of the incoming viral genome, whereas negative-sense single-stranded RNA [(-)ssRNA] viruses carry at infection an RNA replicase that makes multiple translation-competent copies of the incoming viral genome, we reasoned that (+)ssRNA viruses will be more sensitive to the effect of Slfn11 on protein synthesis than (-)ssRNA viruses. To evaluate this hypothesis, we tested the effects of Slfn11 on the replication of a panel of ssRNA viruses in the human glioblastoma cell line A172, which naturally expresses Slfn11. Depletion of Slfn11 significantly increased the replication of (+)ssRNA viruses from the Flavivirus genus, including West Nile virus (WNV), dengue virus (DENV), and Zika virus (ZIKV), but had no significant effect on the replication of the (-)ssRNA viruses vesicular stomatitis virus (VSV) (Rhabdoviridae family) and Rift Valley fever virus (RVFV) (Phenuiviridae family). Quantification of the ratio of genome-containing viral particles to PFU indicated that Slfn11 impairs WNV infectivity. Intriguingly, Slfn11 prevented WNV-induced downregulation of a subset of tRNAs implicated in the translation of 11.8% of the viral polyprotein. Low-abundance tRNAs might promote optimal protein folding and enhance viral infectivity, as previously reported. In summary, this study demonstrates that Slfn11 restricts flavivirus replication by impairing viral infectivity.IMPORTANCE We provide evidence that the cellular protein Schlafen 11 (Slfn11) impairs replication of flaviviruses, including West Nile virus (WNV), dengue virus (DENV), and Zika virus (ZIKV). However, replication of single-stranded negative RNA viruses was not affected. Specifically, Slfn11 decreases the infectivity of WNV potentially by preventing virus-induced modifications of the host tRNA repertoire that could lead to enhanced viral protein folding. Furthermore, we demonstrate that Slfn11 is not the limiting factor of this novel broad antiviral pathway.
Keywords: Schlafen 11; flavivirus; virus restriction factors.
Copyright © 2019 American Society for Microbiology.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

